Global Healthcare Analytical Testing Services Market - Key Trends and Drivers Summarized
Why Are Healthcare Analytical Testing Services Becoming Essential for Ensuring Safety, Compliance, and Innovation in the Medical and Pharmaceutical Industries?
Healthcare Analytical Testing Services have become essential for ensuring the safety, compliance, and innovation needed in the medical and pharmaceutical industries. But why are these services so critical today? In an era marked by rapid advancements in medical technology and pharmaceutical developments, testing services provide crucial data and insights that ensure the efficacy, safety, and regulatory compliance of drugs, medical devices, and biologics. These services include a wide array of testing processes such as bioanalytical testing, stability studies, toxicology testing, and method validation, all of which are necessary to meet stringent regulatory requirements and ensure patient safety.The demand for more rigorous quality control is especially high given the increasing complexity of healthcare products, from personalized medicines and gene therapies to advanced medical devices. Healthcare analytical testing services help pharmaceutical companies identify impurities, assess drug potency, and verify that their products meet international safety and quality standards, such as those set by the FDA, EMA, and other regulatory bodies. As drug development becomes more globalized and new therapeutic modalities emerge, the role of testing services in maintaining product integrity and ensuring compliance has grown exponentially. In addition to safety and compliance, analytical testing also drives innovation by providing the data needed to develop more effective and safer healthcare solutions.
How Are Technological Advancements and Innovations Improving the Accuracy, Speed, and Scope of Healthcare Analytical Testing Services?
Technological advancements are significantly improving the accuracy, speed, and scope of healthcare analytical testing services, making it easier for companies to ensure product safety and regulatory compliance. One of the most transformative innovations is the development of advanced chromatography and mass spectrometry techniques. These technologies allow for highly precise separation and identification of complex compounds in pharmaceutical products, enabling researchers to detect even trace amounts of impurities, contaminants, or active ingredients. High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), and Liquid Chromatography-Mass Spectrometry (LC-MS) are widely used to provide accurate, reproducible results that are critical in drug development and quality control.Another key advancement is the growing use of automated systems and robotics in analytical testing labs. Automation significantly enhances the speed and efficiency of testing processes by reducing manual labor and human error. Robotic systems can handle high-throughput testing, allowing labs to process larger sample volumes in less time, which is especially important for meeting tight deadlines during clinical trials or product launches. This increase in testing speed helps pharmaceutical companies bring drugs and medical devices to market faster while ensuring that all safety and quality checks are completed.
Artificial intelligence (AI) and machine learning (ML) are also being integrated into healthcare analytical testing services to improve data analysis and predictive modeling. AI algorithms can analyze large datasets generated by testing processes and identify trends, patterns, or anomalies that might otherwise go unnoticed. For example, in bioanalytical testing, AI can help identify biomarkers or genetic variations that may impact drug efficacy or safety, enabling more targeted therapeutic development. Machine learning can also improve the accuracy of predictions related to drug stability, shelf life, and performance under various environmental conditions, helping companies optimize product formulations and storage requirements.
The use of next-generation sequencing (NGS) is revolutionizing bioanalytical testing, particularly in the development of biologics, gene therapies, and personalized medicine. NGS allows researchers to perform detailed genetic analysis, enabling a better understanding of how drugs interact with specific genetic profiles. This technology is essential for identifying genetic mutations, assessing patient responses to treatments, and developing therapies tailored to individual patients' needs. As personalized medicine continues to grow, NGS is becoming a cornerstone of healthcare analytical testing services, providing deep insights into the molecular underpinnings of diseases and drug interactions.
Additionally, the development of in-vitro testing technologies and advanced cell-based assays is expanding the scope of healthcare analytical testing services. These methods allow for more accurate assessments of a drug's pharmacological properties and toxicological effects without the need for animal or human testing. In-vitro testing, for example, enables the evaluation of drug absorption, distribution, metabolism, and excretion (ADME), providing critical data on how a drug behaves in the body. This approach reduces the need for animal testing, speeds up the drug development process, and aligns with growing regulatory and ethical demands for more sustainable and humane testing methods.
Why Are Healthcare Analytical Testing Services Critical for Regulatory Compliance, Product Quality, and Patient Safety?
Healthcare Analytical Testing Services are critical for regulatory compliance, product quality, and patient safety because they provide the data needed to verify that healthcare products meet the highest standards of efficacy, safety, and quality. One of the primary reasons these services are so important is their role in ensuring that pharmaceutical and medical device manufacturers comply with stringent regulatory requirements. Regulatory agencies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other global bodies require extensive testing and validation of products before they can be approved for market release. Analytical testing services perform these essential evaluations, including chemical, microbiological, and toxicological analyses, ensuring that products meet regulatory guidelines and are safe for consumer use.Product quality is another critical area where healthcare analytical testing services are indispensable. High-quality testing ensures that drugs, biologics, and medical devices are free from harmful contaminants, impurities, and defects. By conducting stability testing, for instance, analytical services can determine how a drug's potency and safety are affected over time under various environmental conditions, such as temperature, humidity, and light exposure. These insights allow manufacturers to establish proper storage conditions, expiration dates, and packaging requirements, all of which are crucial for maintaining product quality throughout the supply chain. Consistent quality testing helps manufacturers prevent product recalls, safeguard their reputation, and ensure that patients receive safe and effective treatments.
Patient safety is at the core of healthcare analytical testing services. Ensuring that products are free from harmful substances, properly dosed, and effective is essential to protect patients from adverse effects or ineffective treatments. For instance, during bioanalytical testing, labs analyze drug concentrations in biological fluids to ensure that patients are receiving the correct dosage and that the drug is metabolizing as expected. Similarly, toxicology testing evaluates the potential for a drug or medical device to cause harm over short-term or long-term use. Without these rigorous evaluations, patients would be at higher risk for harmful side effects, allergic reactions, or drug interactions.
In addition to ensuring patient safety, analytical testing services play a crucial role in mitigating risks during the drug development process. By identifying impurities or issues early on, testing can prevent costly errors and ensure that clinical trials proceed smoothly. For example, extractables and leachables testing identifies any chemicals that may leach from packaging or manufacturing materials into a pharmaceutical product, which could compromise its safety or efficacy. By performing these tests before a product is released, companies can proactively address potential risks, ensuring that the final product meets all safety standards.
The role of healthcare analytical testing services also extends to post-market surveillance. Even after a drug or device has been approved and released to the public, ongoing testing is often required to monitor long-term safety and efficacy. This ensures that any emerging risks are detected early, and if necessary, corrective actions can be taken, such as issuing recalls or updating product warnings. Analytical testing also helps verify that products continue to meet quality standards over time, especially for treatments that are used over extended periods, such as chronic disease medications or medical implants.
What Factors Are Driving the Growth of the Healthcare Analytical Testing Services Market?
Several key factors are driving the rapid growth of the Healthcare Analytical Testing Services market, including the increasing complexity of drug development, stricter regulatory requirements, the rise of biologics and personalized medicine, advancements in testing technologies, and the trend toward outsourcing analytical services. One of the primary drivers is the growing complexity of pharmaceuticals and medical devices. As more advanced therapies such as biologics, gene therapies, and cell-based treatments enter the market, there is an increasing need for specialized testing services that can handle these complex products. Traditional small-molecule drugs are giving way to large-molecule biologics, which require more sophisticated and sensitive testing methods to ensure safety and efficacy.Stricter regulatory requirements imposed by agencies like the FDA, EMA, and other global bodies are also fueling the demand for healthcare analytical testing services. As governments around the world tighten regulations on drug approvals, companies are required to perform more extensive testing, including bioanalytical testing, stability studies, and toxicology assessments. This has led pharmaceutical companies and medical device manufacturers to increasingly rely on external testing labs to meet these rigorous requirements. The push for more thorough evaluations of potential risks, contaminants, and long-term effects is driving growth in the analytical testing sector.
The rise of biologics and personalized medicine is another significant factor contributing to the expansion of the healthcare analytical testing services market. Biologics, such as monoclonal antibodies and cell-based therapies, require highly specialized testing to ensure product quality and patient safety. Personalized medicine, which tailors treatments to individual genetic profiles, further drives demand for precise and detailed testing to determine how specific patients will respond to therapies. Next-generation sequencing (NGS), biomarker testing, and genetic analysis are increasingly being integrated into analytical testing services to support the development of these cutting-edge treatments.
Advancements in testing technologies are also propelling the growth of the market. New technologies, such as advanced chromatography, mass spectrometry, and next-generation sequencing, are making it easier to detect impurities, quantify active ingredients, and assess drug stability with unprecedented accuracy. Automation and robotics in analytical testing labs are improving throughput and efficiency, allowing companies to process larger volumes of samples while maintaining high standards of accuracy and precision. These innovations are helping testing labs meet the growing demand for faster, more detailed analyses in an increasingly competitive and fast-paced pharmaceutical landscape.
The trend toward outsourcing analytical testing services is another key driver of market growth. Pharmaceutical companies and medical device manufacturers are increasingly outsourcing their testing needs to specialized contract research organizations (CROs) and testing labs. Outsourcing allows companies to access state-of-the-art equipment, specialized expertise, and regulatory knowledge without having to invest in expensive in-house testing capabilities. This approach helps companies reduce costs, shorten development timelines, and ensure that their products meet regulatory standards. As the pharmaceutical industry continues to grow, particularly in emerging markets, the demand for outsourced analytical testing services is expected to rise.
In conclusion, the growth of the Healthcare Analytical Testing Services market is driven by the increasing complexity of drug development, stricter regulatory requirements, advancements in testing technologies, the rise of biologics and personalized medicine, and the trend toward outsourcing. As the pharmaceutical and medical device industries continue to innovate and evolve, healthcare analytical testing services will remain essential for ensuring product safety, regulatory compliance, and overall quality, making them a critical component of the healthcare ecosystem.
Report Scope
The report analyzes the Healthcare Analytical Testing Services market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Bioanalytical Testing Services, Environmental Monitoring Services, Physical Characterization Services, Method Development & Validation, Raw Materials Testing, Batch-Release Testing Services, Stability Testing, Microbial Testing); End-Use (Pharma & Biotech Companies, Medical Device Companies, Clinical Research Organizations (CROs)).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Bioanalytical Testing Services segment, which is expected to reach US$2.6 Billion by 2030 with a CAGR of 8.6%. The Environmental Monitoring Services segment is also set to grow at 8.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.8 Billion in 2024, and China, forecasted to grow at an impressive 13.5% CAGR to reach $2.6 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Healthcare Analytical Testing Services Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Healthcare Analytical Testing Services Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Healthcare Analytical Testing Services Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Charles River Laboratories International, Inc., Envigo, Inc., Eurofins Scientific SE, Exova Group PLC, Intertek Group PLC and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 13 companies featured in this Healthcare Analytical Testing Services market report include:
- Charles River Laboratories International, Inc.
- Envigo, Inc.
- Eurofins Scientific SE
- Exova Group PLC
- Intertek Group PLC
- Laboratory Corporation of America Holdings (LabCorp)
- Merck KgaA
- MPI Research, Inc.
- Pace Analytical Services, Inc.
- Pharmaceutical Product Development LLC
- SGS SA
- Source BioScience PLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Charles River Laboratories International, Inc.
- Envigo, Inc.
- Eurofins Scientific SE
- Exova Group PLC
- Intertek Group PLC
- Laboratory Corporation of America Holdings (LabCorp)
- Merck KgaA
- MPI Research, Inc.
- Pace Analytical Services, Inc.
- Pharmaceutical Product Development LLC
- SGS SA
- Source BioScience PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 232 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
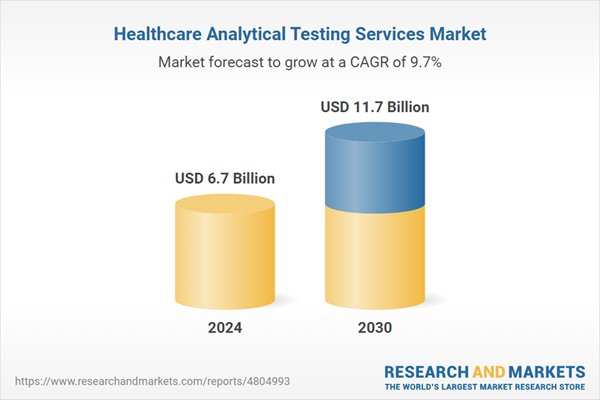
| Estimated Market Value ( USD | $ 6.7 Billion |
| Forecasted Market Value ( USD | $ 11.7 Billion |
| Compound Annual Growth Rate | 9.7% |
| Regions Covered | Global |