Global Pyrogen Testing Market - Key Trends and Drivers Summarized
Pyrogen Testing: Ensuring the Safety of Pharmaceutical and Medical Products
Pyrogen testing is a critical process in the pharmaceutical and medical industries to detect and quantify pyrogens, which are fever-inducing substances that can cause severe reactions in humans and animals. Pyrogens, often bacterial endotoxins, can be introduced into products during manufacturing processes and can lead to life-threatening conditions such as septic shock if not properly identified and removed. Pyrogen testing is essential for ensuring the safety of injectable drugs, vaccines, intravenous (IV) fluids, and medical devices that come into contact with blood or cerebrospinal fluid. By rigorously testing for the presence of pyrogens, manufacturers can prevent harmful contaminants from reaching patients, thereby maintaining product safety and regulatory compliance.How Are Technological Advancements Enhancing Pyrogen Testing Methods?
Technological advancements have significantly improved the accuracy, sensitivity, and efficiency of pyrogen testing methods. Traditional tests, such as the Rabbit Pyrogen Test (RPT) and the Limulus Amebocyte Lysate (LAL) assay, have been the standard for detecting pyrogens. However, recent innovations have led to the development of more sophisticated and ethical alternatives, such as recombinant Factor C (rFC) assays, which do not rely on animal-derived components and offer greater consistency in results. Additionally, the Monocyte Activation Test (MAT) has emerged as a viable alternative, using human blood cells to detect pyrogens, thereby providing a more physiologically relevant test that can detect non-endotoxin pyrogens. The integration of automation and microfluidic technologies into pyrogen testing has also streamlined the testing process, reducing the time required to obtain results and minimizing human error. These technological advancements are driving the adoption of next-generation pyrogen testing methods, ensuring higher levels of safety and efficiency in pharmaceutical and medical product manufacturing.What Are the Key Applications and Benefits of Pyrogen Testing in the Pharmaceutical Industry?
Pyrogen testing is utilized across a wide range of applications within the pharmaceutical and medical device industries, offering numerous benefits that enhance product safety, quality, and regulatory compliance. In the production of injectable drugs and vaccines, pyrogen testing is crucial for detecting bacterial endotoxins that could cause adverse reactions in patients. Medical device manufacturers rely on pyrogen testing to ensure that products such as surgical implants, IV catheters, and dialysis machines are free from harmful contaminants. The testing process is also critical in the quality control of raw materials, such as water used in pharmaceutical manufacturing, to prevent contamination at the source. Additionally, pyrogen testing plays a vital role in the development and approval of new drugs, where regulatory agencies require thorough testing to demonstrate the safety of products before they can be marketed. The primary benefits of pyrogen testing include ensuring patient safety, maintaining product integrity, and meeting stringent regulatory requirements, making it an indispensable practice in the pharmaceutical industry.What Factors Are Driving the Growth in the Pyrogen Testing Market?
The growth in the Pyrogen Testing market is driven by several factors. The increasing demand for biologics, vaccines, and injectable drugs is a significant driver, as these products require rigorous pyrogen testing to ensure safety and efficacy. Technological advancements in testing methods, such as the development of recombinant assays and the adoption of automation, are also propelling market growth by offering more reliable and efficient testing solutions. The rising focus on animal welfare and the push for ethical testing practices are further boosting demand for alternative pyrogen testing methods, such as the Monocyte Activation Test (MAT). Additionally, the expansion of the pharmaceutical and medical device industries in emerging markets is contributing to market growth, as these regions require robust testing infrastructure to meet global standards. The growing emphasis on regulatory compliance and the increasing complexity of pharmaceutical products are also supporting the adoption of advanced pyrogen testing methods. These factors, combined with continuous innovation in testing technologies, are driving the sustained growth of the Pyrogen Testing market.Report Scope
The report analyzes the Pyrogen Testing market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Assays, Kits & Reagents, Services, Instruments); Test Type (LAL Tests, In-Vitro Tests, Rabbit Tests); End-Use (Pharma & Biotech Companies, Medical Device Companies, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Assays, Kits & Reagents segment, which is expected to reach US$1.1 Billion by 2030 with a CAGR of 10.9%. The Services segment is also set to grow at 10.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $326.7 Million in 2024, and China, forecasted to grow at an impressive 14.6% CAGR to reach $498.7 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pyrogen Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pyrogen Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pyrogen Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Associates of Cape Cod, Inc., Charles River Laboratories International, Inc., Ellab A/S, GenScript Biotech Corporation, Hyglos GmbH and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 12 companies featured in this Pyrogen Testing market report include:
- Associates of Cape Cod, Inc.
- Charles River Laboratories International, Inc.
- Ellab A/S
- GenScript Biotech Corporation
- Hyglos GmbH
- Lonza Group AG
- Merck KgaA
- Thermo Fisher Scientific, Inc.
- Wako Chemicals USA, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Associates of Cape Cod, Inc.
- Charles River Laboratories International, Inc.
- Ellab A/S
- GenScript Biotech Corporation
- Hyglos GmbH
- Lonza Group AG
- Merck KgaA
- Thermo Fisher Scientific, Inc.
- Wako Chemicals USA, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 158 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
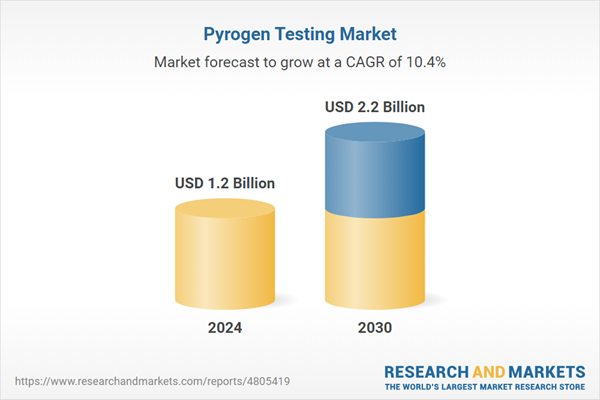
| Estimated Market Value ( USD | $ 1.2 Billion |
| Forecasted Market Value ( USD | $ 2.2 Billion |
| Compound Annual Growth Rate | 10.4% |
| Regions Covered | Global |