Global Lateral Flow Assays Market - Key Trends & Drivers Summarized
Lateral Flow Assays (LFAs) are a widely utilized diagnostic tool in various fields, including medical diagnostics, food safety, and environmental testing. These assays operate on a simple yet effective principle: a sample is applied to a test strip, and the presence of a target analyte is indicated by a visible line on the strip. LFAs are renowned for their rapid results, ease of use, and cost-effectiveness, making them particularly valuable in settings where quick decision-making is crucial. One of the most familiar examples of LFAs is the home pregnancy test. Beyond pregnancy tests, LFAs are employed for detecting infectious diseases, monitoring chronic conditions, and ensuring food and water safety, highlighting their versatility and broad applicability.In recent years, the significance of LFAs has surged where they played a pivotal role in large-scale testing efforts. The ability of LFAs to provide quick and reliable results without the need for sophisticated laboratory equipment made them indispensable in managing the pandemic. Their application extended to detecting various biomarkers, including antigens and antibodies, facilitating widespread and accessible testing. Furthermore, ongoing advancements in LFA technology have enhanced their sensitivity and specificity, enabling the detection of lower concentrations of analytes and reducing the likelihood of false positives and negatives. These improvements have expanded the scope of LFAs, making them suitable for a broader range of diagnostic applications. Advancements in LFAs are also intended to improve these assays on the basis of selectivity, sensitivity and quantification. While test readers offered by various players are finding increasing acceptance to measure intensity of test line of specimen, devices with high level of portability and miniaturization are boosting uptake of handheld lateral flow assay readers. Innovations are also favored by increasing use of unique wavelengths to illuminate samples along with implementation of different sample detection methods like CCD or CMOS detection technology. The availability of advanced test-specific image processing algorithms has improved sample analyte quantification.
The growth in the Lateral Flow Assays market is driven by several factors, including technological advancements, increasing demand for point-of-care testing, and evolving consumer behavior. Technological innovations, such as the integration of smartphone-based readers and digital platforms, have significantly enhanced the functionality and user experience of LFAs. These advancements enable better data management, remote monitoring, and real-time result sharing with healthcare providers. The rising prevalence of chronic and infectious diseases has also spurred demand for rapid and reliable diagnostic tools that can be used outside traditional healthcare settings. Consumer behavior is shifting towards more proactive health management, with individuals seeking convenient and accessible testing options. Additionally, regulatory support and government initiatives promoting the development and adoption of point-of-care diagnostics are accelerating market growth. Collaborations between diagnostic companies and research institutions are fostering innovation, leading to the development of next-generation LFAs with improved performance and expanded capabilities. These factors collectively ensure the robust growth and ongoing evolution of the LFA market.
Report Scope
The report analyzes the Lateral Flow Assays market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Kits & Reagents, Lateral Flow Readers); Application (Clinical Testing, Veterinary Diagnostics, Other Applications); End-Use (Hospitals & Clinics, Diagnostic Laboratories, Pharma & Biotech Companies, Other End-Uses).
- Geographic Regions/Countries: World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Kits & Reagents segment, which is expected to reach US$12.3 Billion by 2030 with a CAGR of 5.4%. The Lateral Flow Readers segment is also set to grow at 4.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4 Billion in 2024, and China, forecasted to grow at an impressive 7.7% CAGR to reach $1.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Lateral Flow Assays Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Lateral Flow Assays Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Lateral Flow Assays Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Merck KGaA, Hologic, Inc., Danaher Corporation, Polysciences, Inc., Iul, S.A. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 55 companies featured in this Lateral Flow Assays market report include:
- Merck KGaA
- Hologic, Inc.
- Danaher Corporation
- Polysciences, Inc.
- Iul, S.A.
- ANP Technologies, Inc.
- Bioporto Diagnostics A/S
- Abingdon Health Ltd.
- BBI Solutions
- IMMY
- Creative Diagnostics
- Pribolab Pte., Ltd.
- DCN Diagnostics
- Microcoat Biotechnologie GmbH
- DIALUNOX GmbH
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Merck KGaA
- Hologic, Inc.
- Danaher Corporation
- Polysciences, Inc.
- Iul, S.A.
- ANP Technologies, Inc.
- Bioporto Diagnostics A/S
- Abingdon Health Ltd.
- BBI Solutions
- IMMY
- Creative Diagnostics
- Pribolab Pte., Ltd.
- DCN Diagnostics
- Microcoat Biotechnologie GmbH
- DIALUNOX GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 461 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
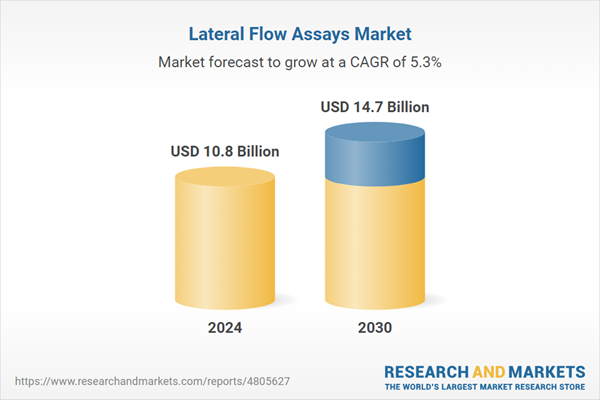
| Estimated Market Value ( USD | $ 10.8 Billion |
| Forecasted Market Value ( USD | $ 14.7 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |