Continuous developments in recombinant DNA technology are anticipated to enhance the efficiency of gene therapy in the coming years. Hence, ongoing progresses in recombinant DNA technology are anticipated to expand the number of ongoing clinical trials for gene therapy. Primarily, these advancements are taking place in the context of various gene-editing tools and expression systems to augment the R&D for products. The advent of CRISPR/Cas9 nuclease, ZFN, and TALEN allows easy & precise genome editing. As a result, in recent times, the gene-editing space has witnessed a substantial number of research activities, which, in turn, is expected to influence the growth of the gene therapy market.
The growth of the gene therapy market is expected to be majorly benefitted from the increasing prevalence of cancer. The ongoing increase in cancer patients and related death per year emphasizes the essential for the development of robust treatment solutions. In 2020, there were around 18.1 million new cases of cancer worldwide. 9.3 million of these cases involved men, while 8.8 million involved women. Continuing developments in tumor genetic studies have delivered substantial information about cancer-related molecular signatures, which in turn, is expected to support ongoing clinical trials for cancer therapeutics.
With rising demand for robust disease treatment therapies, companies have focused their efforts to accelerate R&D for effective genetic therapies that target the cause of disease at a genomic level. . Furthermore, the U.S. FDA provides constant support for innovations in this sector via a number of policies with regard to product manufacturing. In January 2020, the agency released six final guidelines on the manufacturing and clinical development of safe and efficient products.
Furthermore, facility expansion for cell and gene therapies is one of the major factors driving the gene therapy market growth. Several in-house facilities and CDMOs for gene therapy manufacturing have begun investing to enhance their production capacity, which, in turn, is anticipated to create lucrative opportunities for market players. For instance, in April 2022, the FDA approved commercial licensure approval to Novartis for its Durham, N.C. site. This approval permits the 170,000 square-foot facility to make, test, and issue commercial Zolgensma, as well as manufacture therapy products for current & upcoming clinical trials.
Gene Therapy Market Report Highlights
- The AAV segment shows a significant revenue contribution of 22% in 2023. Several biopharma companies are offering their viral vector platform for the development of AAV-based gene therapy product.
- By indication, the spinal muscular atrophy (SMA) segment dominated the market in 2023 with a share of 46.8%. Although SMA is a rare disorder, it is one of the most common fatal inherited diseases of infancy.
- The Beta-Thalassemia Major/SCD segment is anticipated to register the fastest CAGR of 38.3% over the forecast period. Gene therapy for SCD and β-thalassemia is based on transplantation of gene-modified hematopoietic stem cells.
- North America dominated the market in 2023 with the largest revenue share of 65.2% in 2023. This region is expected to become the largest routine manufacturer of gene therapy in terms of the number of approvals and revenue generated during the forecast period.
- Europe is estimated to be the fastest-growing regional segment from 2024 to 2030. This is attributed to its large population with unmet medical needs and increasing demand for novel technologies in the treatment of rare but increasingly prevalent diseases.
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned
- Amgen Inc.
- Novartis AG
- F. Hoffmann-La Roche
- Gilead Sciences, Inc.
- bluebird bio, Inc.
- Bristol-Myers Squibb Company
- Legend Biotech.
- BioMarin.
- uniQure N.V.
- Merck & Co.
- Sarepta Therapeutics, Inc.
- Krystal Biotech, Inc
- CRISPR Therapeutics.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 120 |
| Published | July 2024 |
| Forecast Period | 2023 - 2030 |
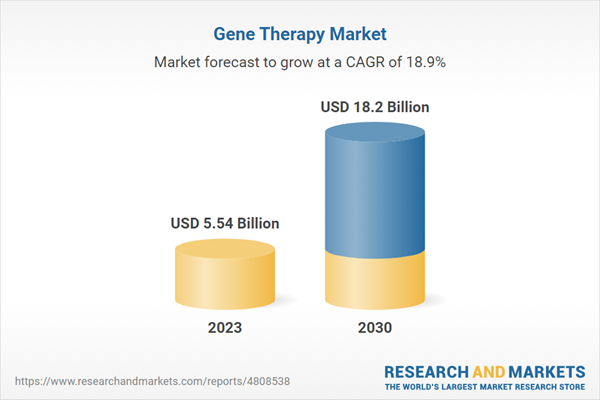
| Estimated Market Value ( USD | $ 5.54 Billion |
| Forecasted Market Value ( USD | $ 18.2 Billion |
| Compound Annual Growth Rate | 18.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |