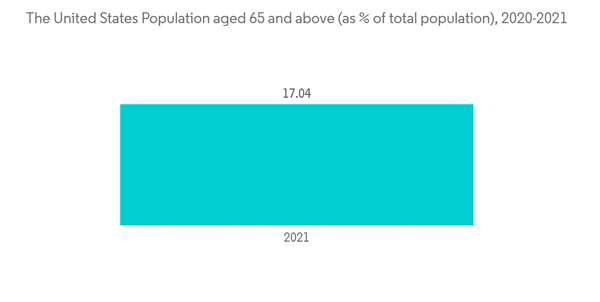The COVID-19 pandemic has substantially impacted the allergy-relieving eye drops market. The strict lockdowns and government regulations slowed the spread of COVID-19, resulting in supply chain disruptions. The ophthalmic care system was affected severely during the pandemic due to lockdown restrictions. For instance, according to an article published by PLOS One journal in March 2022, an observational study was conducted in a community hospital in Taiwan, which showed a significant decrease in the number of ophthalmic outpatients during the pandemic. Furthermore, according to an article published by PubMed in December 2020, a study conducted in a COVID-19 hotspot in the United States showed that the total number of eye-specific visits dropped considerably during the pandemic compared to prior years, and patients were found to be more reluctant to seek eye care. Thus, the COVID-19 outbreak affected the market's growth adversely in its preliminary phase; however, the market has regained its traction as the pandemic has subsided.
The rise in prevalence and incidence rates of eye-related disorders, increased geriatric population, and growing research and development in eye allergy medication are expected to boost the allergy-relieving eye drops market during the forecast period.
Eye Allergy has been increasing in recent years. According to an article published by PubMed in October 2020, the prevalence of ocular allergies has been growing worldwide for the past several decades. The geographical distribution and hot spots of rhinoconjunctivitis have been documented in a global survey by the International Study of Asthma and Allergies in Childhood (ISAAC). According to ISAAC, Africa, Latin America, and Japan have a considerably high prevalence of rhinoconjunctivitis. Furthermore, according to the data by the World Bank Group in 2021, the population aged 65 and above (as a percentage of the total population) increased from 6.57 percent in 2020 to 6.78 percent in 2021; the rising geriatric population will be accompanied by more eye problem which will increase the usage of allergy relieving eye drops. Hence, it is expected to enhance the market growth.
In addition, new product launches and strategic activities by major players in the market are positively affecting the growth of the studied market. For example, in September 2020, Dr. Reddy's Laboratories launched an over-the-counter (OTC) eye allergy drop Olopatadine Hydrochloride Ophthalmic Solution, in the United States market. Thus, owing to the product launches by key market players, the studied market is expected to have significant growth over the forecast period.
Therefore, owing to the factors above, the studied market is anticipated to witness growth over the analysis period. However, side effects from allergy-relieving eye drops, including irritation, headache, and others, have been restraining the overall development of this market.
Allergy Relieving Eye Drops Market Trends
Antihistamines are Expected to Register a High Growth Rate Over the Forecast Period
Antihistamines are found to block histamine release from histamine-1 receptors and treat the symptoms of an allergic reaction, such as edema (swelling), itch, inflammation (redness), and watery eyes. Published Eye edema is commonly caused by unhealthy cornea before surgery. According to an article by WebMD LLC. on January 2022, antihistamines help reduce or block histamines, stopping allergy symptoms. The antihistamines work well to relieve symptoms of different allergies, including seasonal (hay fever), indoor, and food allergies. According to an article published by BMJ in April 2022, a cross-sectional study was performed, which showed that the prevalence of Diabetic macular edema (DME) among the patients was found to be very high.Subsequently, any procedure within the eye (such as cataract, glaucoma, or retinal surgery) can cause the cornea to fail and develop edema. Therefore antihistamines effectively treat edema, and the market is expected to increase over the forecast period.
Furthermore, critical developments in the market are positively affecting the segment's growth. For instance, in February 2021, Bausch + Lomb, a leading global eye health business of Bausch Health Companies Inc., announced the United States launch of Alaway Preservative Free (ketotifen fumarate ophthalmic solution 0.035%) antihistamine eye drops, which is the first and only over-the-counter (OTC) preservative-free antihistamine eye itch relief drop approved by FDA.
Therefore, the antihistamines segment is expected to witness a high growth rate over the forecast period due to the abovementioned factors.
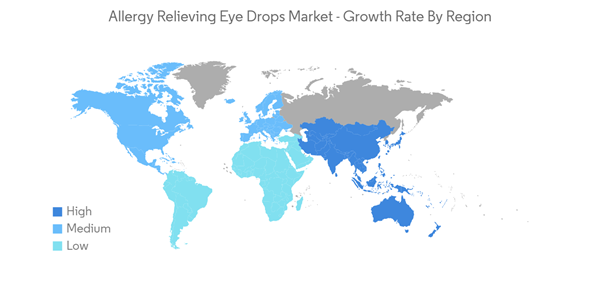
North America is Dominating the Allergy Relieving Eye Drops Market
North America is dominating the market owing to factors such as the rising geriatric population, easy availability of products, and high awareness among patients about allergy care in the region. North America includes the United States (US), Canada, and Mexico. The United States and Canada have developed healthcare systems and also spend huge amounts in research and development for the same. Therefore, as a result, most of the global treatments are available in this region.According to the data by CDC in June 2020, an estimated 12 million people 40 years and over in the United States have vision impairment, including 1 million who are blind, 3 million who have vision impairment after correction, and 8 million who have vision impairment due to uncorrected refractive error. Hence, with the rising prevalence of eye disorders in the United States, the adoption of allergy relieving eye drops is also expected to increase during the forecast period.
Furthermore, key developments in the market are positively affecting the growth of the segment. For instance, in September 2020, the United States FDA approved an over-the-counter preservative-free eye drop for itchy eyes due to certain eye allergies, according to a Bausch Health press release, Alaway preservative-free (ketotifen fumarate) ophthalmic solution 0.035% antihistamine eye drops are approved to relieve itchy eyes caused by pollen, ragweed, grass, animal hair, and dander.
Therefore, owing to the aforesaid factors the growth of the studied market is anticipated in the North America Region.
Allergy Relieving Eye Drops Market Competitor Analysis
The allergy-relieving eye drops market is fragmented due to the presence of several companies operating globally and regionally. The competitive landscape includes an analysis of a few international as well as local companies which hold market shares and are well known, including AbbVie Inc. (Allergan Plc), Johnson & Johnson, Pfizer Inc., F. Hoffmann-La Roche Ltd, Novartis AG, Bausch Health Companies Inc, Regeneron Pharmaceuticals, and Santen Pharmaceutical Co among others.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc. (Allergan Plc)
- Johnson & Johnson
- Pfizer Inc.
- F. Hoffmann-La Roche Ltd
- Novartis AG
- Bausch Health Companies Inc
- Regeneron Pharmaceuticals
- Santen Pharmaceutical Co