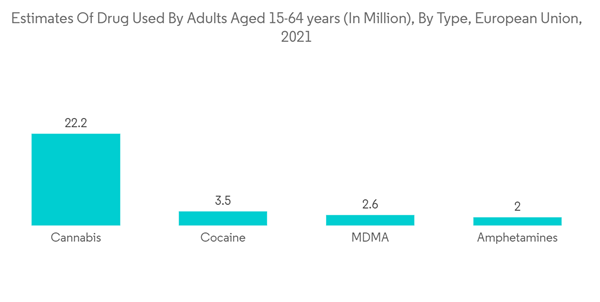The lockdown and restrictions amid the pandemic canceled or delayed the diagnosis and treatment of non-COVID and non-urgent medical conditions including substance-addicted and alcohol-addicted patients. For instance, a study published in the JAMA Network in November 2021 stated that federal and state governments implemented temporary strategies for providing access to opioid use disorder (OUD) treatment during the COVID-19 pandemic, in the United States. The study further stated that all the states and Washington, DC of the United States adopted at least one telehealth policy to improvise access to OUD treatment for new patients. Thus, there was anan increase in demand for substance abuse treatment amid the ongoing COVID-19 pandemic. Although in the post-pandemic, numbers of COVID-19 patients decreased gradually which led the market to grow at a normal pace. Thus, the COVID-19 outbreak had a moderately impacted the market's growth in its preliminary phase. Moreover, the market is expected to grow further at a stable pace with the increasing demand for the susbtance abuse treatment, globally.
Further, the substance abuse treatment market is expected to grow with the rising drug awareness campaigns and prevention programs and growth in the number of addictions among the population.
The rising awareness of substance abuse, drug addiction and their prevention and management with the support of private and public bodies are expected to create more demand for substance abuse treatment. For instance, in December 2021, the Indian government launched the "Drug-Free India" campaign and issued directions for curbing drug abuse in the Indian populace. Likewise, in October 2021, the Centers for Disease Control and Prevention (CDC) launched four complementary education campaigns intended to reach young adults ages 18-34 years aimed at preventing drug overdose deaths. The campaigns provide information about the prevalence and dangers of fentanyl, the risks and consequences of mixing drugs, the life-saving power of naloxone, and the importance of reducing the stigma around drug use to support treatment and recovery. Such campaigns and initiatives are increasing awareness regarding substance abuse treatment among the addicted population, thereby contributing to the market's growth.
Furthermore, the key developments by the market players in substance abuse treatment are expected to boost market growth. For instance, in June 2021, INDIVIOR PLC and AELIS FARMA announced a strategic partnership and option-license agreement to address the significant repercussions of cannabis use disorders (CUD), including cannabis-induced psychosis (CIP). Further, in May 2021, JB Chemicals & Pharmaceuticals Ltd (JBCPL) launched NOSMOK prescription medicated nicotine lozenges as part of a 360 Tobacco Cessation initiative on 'World No Tobacco Day' that will aid in reducing the urge to consume tobacco.
Therefore, with the growth factors such as rising drug awareness, and the key developments by the market players, the studied market is anticipated to witness growth over the analysis period. However, the reluctance of individuals for accessing treatment and discontinuation of behavioral therapies, and poor treatment compliance in some countries are likely to impede the market growth.
Substance Abuse Treatment Market Trends
Tobacco/Nicotine Addiction Treatment Segment is Expected to Witness Significant Growth Over the Forecast Period
By treatment type, the tobacco/nicotine addiction treatment segment is anticipated to witness lucrative growth. Tobacco/nicotine addiction treatment includes pharmacotherapies and replacement therapies to curb the addiction to tobacco and nicotine. Tobacco and its usage through consumption and smoking are harmful, as they kill half of its users globally, as stated by the World Health Organization (WHO). In May 2022, WHO stated that tobacco claims the lives of more than 8 million people every year. More than 7 million deaths are caused by direct tobacco use, and over 1.2 million non-smokers lose their lives due to exposure to second-hand smoke. Such a vast number of tobacco users and damage caused by tobacco addiction is anticipated to boost the demand for Tobacco/nicotine addiction treatment which would bolster the growth of the segment.Furthermore, several market players are engaged in advancing their offerings for tobacco/nicotine deaddiction with their smoking cessation therapies and treatment which are undergoing clinical trials. For instance, in December 2021, NFL Biosciences launched the Phase II/III clinical trial to assess the efficacy and safety of its NFL-101 smoking cessation treatment. It was approved by the Human Research Ethics Committee (HREC) in Australia in June 2021, and by the French National Agency for the Safety of Medicines and Health Products (ANSM), in September 2021. Similarly, in September 2021, Mydecine Innovations Group launched a Phase 2/3 clinical trial for smoking cessation for its MYCO-001 compound, following up on its recent announcement of 5-year cooperation with Johns Hopkins University's research institute. MYCO-001 compound is inteneded for treatment of nicotine addiction. Such active clinical trials would pave way for the market entry of these tobacco/nicotine addiction treatments or therapies which would further boost the growth of the segment.
Therefore, the tobacco/nicotine addiction treatment segment is expected to witness significant growth over the forecast period due to the abovementioned factors, including the high numbers of tobacco users and the active clinical trials of tobacco/nicotine addiction therapies.
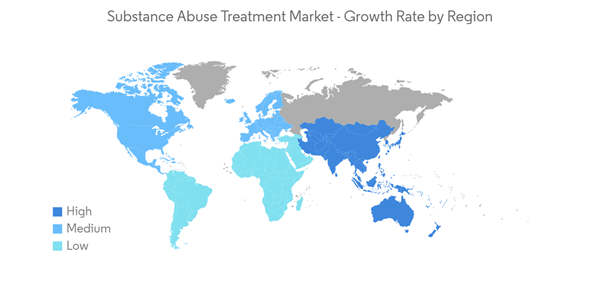
North America is Expected to Witness Significant Growth Over the Forecast Period
The substance abuse treatment market in North America is expected to grow significantly during the forecast period with increasing overuse of tobacco and an increasing number of government initiatives aimed at reducing substance abuse. For instance, as per the data published by the Substance Abuse and Mental Health Services Administration (SAMHSA) in 2021, over 61,203 people aged 12 years and above were reported to have consumed illicit drugs in the year 2021, in the United States. Further, according to the data published by the Canadian Mental Health Association (CMHA), it was estimated that over 21% (over 6 million Canadian individuals) of the Canadian population would meet the criteria for drug addiction in their life. Such a prevalent addiction population within the country is anticipated to create more demand for addiction treatments, in turn, driving the market growth in the region.Furthermore, various initiatives and funding are being implemented by the United States government to monitor the symptoms and determine drug abuse among the American population. For instance, as per a February 2022 update, Texas and some of its largest counties received USD 1.17 billion as an opioid relief fund as part of a nationwide settlement from three large pharmaceutical distribution companies. Moreover, in February 2022, the United States Department of Human Health and Services announced a USD 10 million Substance Abuse and Mental Health Services Administration (SAMHSA) grant program addressing the health needs of pregnant women and children affected by substance use. Such programs are anticipated to drive the demand for substance abuse treatment in the United States, thereby driving market growth in the North American region.
Additionally, the key developments by the market players in substance abuse treatment are expected to boost market growth in the region. For instance, in August 2022, B.More Inc. received the USFDA approval for its Investigational New Drug (IND) application for its synthetic psilocybin (SYNP-101) which is intended for the management of the alcohol use disorder (AUD). Further, in January 2022, ANANDA Scientific Inc. received USFDA approval for its IND application for the clinical trial evaluation of its drug, Nantheia ATL5. Nantheia ATL5 is an investigational drug that uses cannabidiol (CBD) in ANANDA's proprietary delivery technology and is intended to be used as an adjunctive treatment for opioid use disorder. The study would be done at the Jane and Terry Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles, the United States.
Therefore, owing to the aforesaid factors, including the high drug addictions, de-addiction initiatives and key developments by the key players, the studied market is anticipated to grow further in the North America Region.
Substance Abuse Treatment Industry Overview
The substance abuse treatment market is competitive in nature due to the presence of several companies operating globally as well as regionally. The key players operating in the market include Alkermes PLC, Mallinckrodt LLC, Cipla Ltd, GlaxoSmithKline PLC, and Indivior PLC.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alkermes PLC
- Cipla Ltd
- GlaxoSmithKline PLC
- Mallinckrodt LLC
- Indivior PLC
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd
- Orexo AB
- Apotex
- Opiant Pharmaceuticals Inc.
- Apotex
- BioCorRx Inc.
- Opiant Pharmaceuticals Inc.
- Purdue Pharma LP
- Novartis AG (Sandoz)