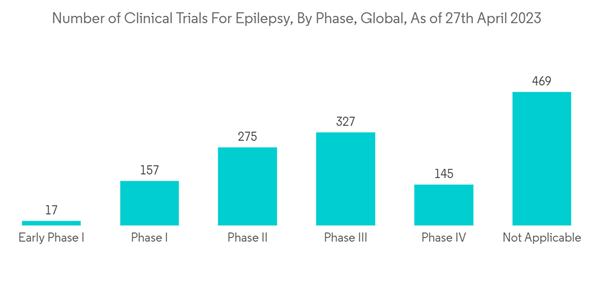
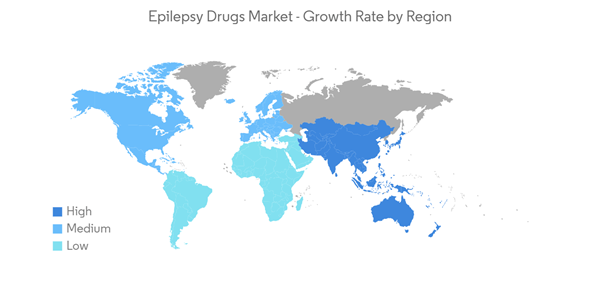
The COVID-19 outbreak significantly impacted the market's growth as several neurology activities, such as medical training and research, were disrupted due to the pandemic. For instance, an article published in the Journal of the International League Against Epilepsy in October 2021 stated that 22.8% of people with epilepsy (PWE) and 27.5% of caregivers reported an increase in seizure frequency, with difficulty in accessing medication and health care professionals reported as barriers to care. Of all respondents, 57.1% of PWE and 21.5% of caregivers had severe psychological distress, significantly higher among PWE than caregivers. Thus, COVID-19 significantly impacted the market's growth in the initial phase. However, owing to the product approvals, the market is anticipated to gain traction over the coming years. For instance, in August 2021, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) approved a new indication for GW Pharmaceuticals's cannabidiol as an adjunctive treatment of seizures associated with tuberous sclerosis complex (TSC) for patients two years of age and older. Thus, the market is expected to grow over the forecast period.
Significant factors contributing to the growth of the epilepsy drugs market include the rise in new drug approvals over the past few years and increasing cases of epilepsy across the globe. As per the World Health Organization's February 2023 update, around 50 million people worldwide suffer from epilepsy, making it one of the most common neurological diseases globally. Nearly 80% of people with epilepsy live in low and middle-income countries. Up to 70% of people with epilepsy could live seizure-free if properly diagnosed and treated. Such a high burden of epilepsy creates the need for treatment and thus drives the market's growth.
Additionally, the increasing launch of drugs for the treatment of epilepsy by market players at affordable prices is also contributing to the growth of the market. For instance, in March 2021, India-based Alkem Laboratories launched Brivasure, an affordable anti-epileptic drug for treating epilepsy in India. Also, in February 2021, Sun Pharmaceutical Industries reported its plans to introduce a complete range of anti-epilepsy drugs, including Brivaracetam, in India at an affordable price.
Furthermore, rising awareness levels about epilepsy and strategic alliances are also anticipated to stimulate market growth. For instance, in November 2021, in the United States, November is celebrated as National Epilepsy Awareness Month (NEAM). Every year, the Epilepsy Foundation, in collaboration with the Epilepsy Foundation, launches the #RemoveTheFilter social media campaign to reduce fear surrounding epilepsy and bring hope to those facing challenges. Such awareness campaigns are also expected to contribute to the market's growth.
Thus, owing to the abovementioned factors, the market's growth is expected to accelerate over the coming years. However, side effects associated with drugs and the recent expiration of patents on major drugs may impede the market's growth.
Epilepsy Drugs Market Trends
Second Generation Anti-epileptics is Expected to Hold a Significant Market Share Over the Forecast Period
Second-generation anti-epileptics drugs are expected to hold a significant share owing to several advantages, such as reduced drug-drug interactions, fewer life-threatening adverse events, and a less negative impact on cognitive functions. Also, the rising cases of epilepsy will boost the demand for drugs in the market segment. Furthermore, according to the article published in Neurological Science in January 2021, there are around 500,000 epilepsy patients (prevalence rate: 8.5/1000), with an annual incidence rate of 33.1 new cases per 100,000 people. While the rate of acute symptomatic seizures is 29-39 per 100,000 people per year, the rate of isolated unprovoked seizures is around 61 per 100,000. It is consistently higher than the rate of epilepsy. Similarly, the World Health Organization (WHO) published a report in April 2022 stating that 100% of countries are expected to include at least one functioning awareness campaign or advocacy program for neurological disorders by 2031. Such initiatives by the renowned organization are expected to drive the market in the coming future.Additionally, the launch of second-line drugs for the treatment of epilepsy by the various market players is propelling the growth of the segment. Also, in February 2021, Dr. Reddy's Laboratories launched Vigabatrin tablets, an antiepileptic drug, in the United States market after receiving approval from the United States Food and Drug Administration.
Besides, the market players are also acquiring other firms to expand their product portfolio. For instance, in January 2022, UCB acquired Zogenix for USD 1.9 billion to bolster its epilepsy portfolio by adding Fintepla (fenfluramine). It is a marketed drug for treating seizures associated with Dravet syndrome, a rare form of childhood epilepsy. This deal continues to expand UCB's extensive therapeutic offering in the epilepsy market, having gained a newly marketed product for patients with rare and difficult-to-treat pediatric orphan epilepsy syndromes.
Hence, owing to the new product launches and acceptance of second-generation epilepsy drugs, the segment is expected to grow over the forecast period.
North America is Expected to Hold Significant Share over the Forecast Period
The North American region is expected to show significant growth over the forecast period owing to the increasing burden of epilepsy coupled with the market players' strategic initiatives, awareness campaigns, and launches of new products. In November 2022, a press release by the CDC stated that epilepsy affects about 3.4 million Americans. About 1 out of 10 people may suffer from a seizure during their lifetime. There are over 260 epilepsy centers in the United States. Healthcare providers at these centers specialize in managing epilepsy and provide expert care to children and adults. Thus, the prevalence of epilepsy in the region is expected to increase the demand for its treatment and, thus, increase the market growth.The approval of drugs for different age populations is also boosting the market's growth. For instance, in August 2021, UCB received United States Food and Drug Administration (FDA) approval for an expanded indication for BRIVIACT (brivaracetam) CV tablets, oral solution, and injection to treat partial-onset seizures in patients as young as one month of age. It is the first time the IV formulation of BRIVIACT will be available for pediatric patients when oral administration is not feasible. It is one of the first IV formulations approved to treat partial-onset seizures in children one month and older (nearly 7 years).
Furthermore, various organizations initiated research in the region. For instance, in October 2022, Avicanna Inc. expanded its research collaboration in epilepsy with a new collaboration with the University of Toronto. It is to explore the efficacy of Avicanna's proprietary formulations in pre-clinical models for epilepsy. Thus, the market is expected to grow over the forecast period due to the abovementioned factors.
Epilepsy Drugs Industry Overview
The market studied is a consolidated market owing to the presence of a few major market players. Therefore, most key players focus on expanding their businesses to increase their market share. They are also adopting specific strategies, such as new product development, mergers, and acquisitions. Some players operating in the market are Abbott Laboratories, GlaxoSmithKline PLC, Johnson & Johnson, Novartis AG, Pfizer, Inc., Sanofi SA, Sunovion Pharmaceuticals Inc., and Takeda Pharmaceutical Company Limited, and UCB S.A., among others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- GlaxoSmithKline PLC
- Johnson & Johnson
- Novartis AG
- Pfizer, Inc.
- Sanofi SA
- Takeda Pharmaceutical Company Limited
- Sunovion Pharmaceuticals Inc.
- UCB S.A.,
- Sun Pharmaceutical Industries Limited
- GW Pharmaceuticals plc
- H. Lundbeck A/S
- Alkem Labs.
- Eisai Co., Ltd.