The COVID-19 pandemic is an unprecedented health concern and adversely affects various surgical procedures. Due to regulatory authorities' strict guidance to prevent any non-emergent surgeries, the volume of surgeries has drastically decreased throughout the pandemic. For instance, according to the study published in October 2021, by the National Library of Medicine globally, there has been a 42.8% decrease in general surgery admissions. Thus, the reduction in surgical procedures during the COVID-19 pandemic affected the market's growth. However, the market is gradually stabilizing as COVID-19 cases are declining the number of heart procedures and operations being performed is increasing thus boosting the growth of the transcatheter pulmonary valve market.
Certain factors that are propelling the market growth are the growing burden of congenital heart disease and the rising awareness of transcatheter pulmonary valve therapy. The rising company activities in developing and launching products as well as increasing product approvals are all expected to increase the market growth over the forecast period. For instance, According to CDC, in February 2022, congenital heart disease (CHD) affects nearly 1% of or about 40,000 births per year in the United States. Additionally, 15% of CHDs are associated with genetic conditions and about 20% to 30% of people with a CHD have other physical problems or developmental or cognitive disorders. Children with CHD are about 50% more likely to receive special education services compared to children without birth defects. Thus, an increase in the CHD burden is expected to drive the growth of the studied market.
Additionally, the advancements in technology and increasing product approvals, along with partnerships and acquisitions by key players, are aiding the market's growth. For instance, in March 2021, Medtronic plc received U.S. FDA approval for its Harmony Transcatheter Pulmonary Valve (TPV), the first minimally invasive therapy created to treat patients with a specific type of congenital heart defect of the right ventricle (RV), one of the four chambers of the heart, which makes it difficult for blood to travel from the heart to the lungs. The Harmony TPV, which is placed inside a patient's native anatomy during a catheter-based procedure, was designated as a Breakthrough Therapy under FDA's Breakthrough Device Designation (BDD) program, an approval pathway intended to help patients receive more timely access to certain life-saving technologies. Such technological advancements are predicted to boost the transcatheter pulmonary valve demand and enhance market growth further.
Therefore, owing to the aforementioned factors, the studied market is anticipated to witness growth over the forecast period. However, the availability of alternative technologies and stringent regulations are expected to impede market growth over the forecast period.
Transcatheter Pulmonary Valve Market Trends
Balloon-Expanded Transcatheter Valve Segment is Expected to Witness a Significant Growth Over the Forecast Period
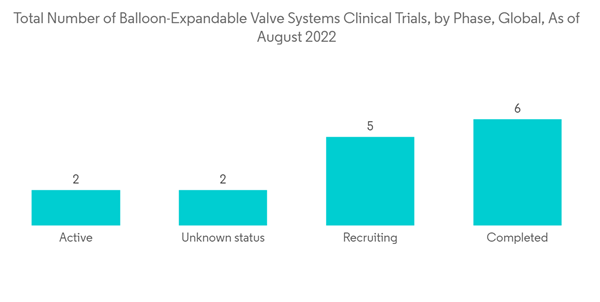
The Balloon-Expanded Transcatheter Valve segment is expected to witness significant growth over the forecast period owing to the factors such as its high adoption rate and rising development of new products. In addition, the growing interest of manufacturers in balloon-expanded technology and rising awareness among the population is also contributing to the growth of the studied market. The high accuracy and precision of the results using this technology are expected to increase segment growth over the forecast period. For instance, according to an article published by the Frontiers Cardiovascular Medicines journal, in March 2022, it has been observed that the burden of rheumatic heart disease (RHD) and patients with pure aortic regurgitation (AR) is increasing in emerging and industrialized countries. This increases the need to extend transcatheter treatments to patients with non-calcified regurgitant aortic valves. Thus, the demand for transcatheter in treating a patient with pulmonary valve defects, thereby propelling the market growth.Additionally, according to an article published by the Elsevier JACC: Asia journal, in September 2021, due to the increasing burden of bicuspid aortic valves in some Asian countries, the new generation balloon-expandable valve (BEV) is found to be an alternative for treating patients with aortic valve disorders. In addition, as per the same source, it has been observed that anomaly, deeply calcified, bigger annular bicuspid aortic valves (BAVs), which were deemed ineligible for transcatheter aortic valve replacement (TAVR) due to a high risk of paravalvular leak (PVL), coronary artery obstruction, permanent pacemaker implantation (PPI), and aortic rupture, might be safely treated with versatile procedures applied to balloon-expandable valve (BEV). Thus, the increasing usage of the balloon-expandable valve in treating patients with aortic regurgitation and bicuspid aortic valve disorders is expected to increase the adoption and demand for balloon-expanded transcatheter valves, thereby fueling market growth.
Thus, owing to the factors above, the studied market is expected to grow during the forecast period.
North America is Expected to Hold a Significant Share in the Market and Expected to do Same in the Forecast Period
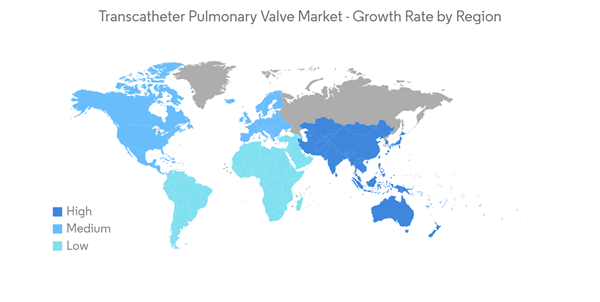
North America is expected to hold a significant share in the transcatheter pulmonary valve market, owing to the presence of a well-developed healthcare sector, along with the increasing prevalence of disorders such as pulmonary stenosis and regurgitation. In addition, rising awareness, growing demand for innovative heart valves, and increasing healthcare expenditure are expected to drive market growth over the forecast period. For instance, according to the Organization for Economic Co-operation and Development (OECD), in June 2022, United States healthcare spending, in 2021 was 17.8% of the total GDP of the country. Additionally, as per the data published by the Centers for Medicare & Medicaid Services, in March 2022, it has been observed that the annual growth in national health spending is expected to average 5.1% over 2021-2030. Thus, the rising health spending in the country is expected to increase company activities and government initiatives in developing efficient transcatheter pulmonary valves, thereby propelling the market growthFurthermore, the rising burden of heart valve disorders, congenital heart disease, and aortic regurgitation among the population is expected to drive market growth over the forecast period. For instance, according to an article published by StatPearls, in April 2022, the prevalence of aortic regurgitation in the United States was between 4.9% and 10%. In addition, as per the same source, it has been observed that men (13%) are more likely to be affected by aortic regurgitation than women (8.5%). Similarly, according to the 2022 statistics published by the Centers for Disease Control and Prevention (CDC), CHD affects nearly 1% or about 40,000 births per year in the United States. Thus, the increasing prevalence of aortic regurgitation is expected to increase the demand for effective treatment which in turn is anticipated to augment the market growth over the forecast period.
Moreover, the rising company’s focus on research and development activities as well as increasing product approvals in the region is expected to increase the market growth over the forecast period. For instance, in August 2021, the United States Food and Drug Administration (FDA) approved Medtronic’s Evolut FX TAVR system, the newest-generation, self-expanding transcatheter aortic valve replacement (TAVR) system, to enhance ease of use and provide greater precision and control throughout the symptomatic severe aortic stenosis procedure. The Evolut technology is equipped with gold markers built into the frame to provide implanters with direct visualization of depth and valve leaflet location during implant. Also, in March 2021, the United States Food and Drug Administration approved Harmony Transcatheter Pulmonary Valve (TPV) System, a non-surgical heart valve to treat pediatric and adult patients with a native or surgically repaired right ventricular outflow tract (RVOT). The device is intended to improve blood flow to the lungs in patients with severe pulmonary valve regurgitation without open-heart surgery.
Thus, owing to the aforementioned factors, the studied market is expected to grow in the region over the forecast period.
Transcatheter Pulmonary Valve Market Competitor Analysis
The transcatheter pulmonary valve market is moderate in nature due to the presence of companies operating globally as well as regionally. The competitive landscape includes an analysis of international and local companies that hold market shares and are well-known, including Boston Scientific Corporation, Braile Biomedica, Artivion, Inc, Inc., Edwards Lifesciences Corporation, JenaValve Technology, Inc, LivaNova PLC, Medtronic PLC, and Venus Medtech.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Boston Scientific Corporation
- Braile Biomedica
- Artivion, Inc
- Edwards Lifesciences Corporation
- JenaValve Technology, Inc
- LivaNova PLC
- Medtronic PLC
- Venus Medtech