COVID-19 is expected to drive the growth of the recombinant hormone market. The increased risk for diseases like diabetes during the COVID-19 outbreak is expected to drive the demand for recombinant hormones like insulin in the market. For instance, in February 2022, Massachusetts General Hospital stated that, in COVID-19 hospital admissions worldwide, high rates of newly diagnosed Diabetic Mellitus (NDDM) had been recorded. This high rate of new diabetes cases increases the need for recombinant insulin. Thus, the outbreak of COVID-19 is expected to drive the growth of the recombinant hormone market during the pandemic period. However, as the pandemic has been subsidized, the market is expected to project stable growth due to the application of recombinant hormones in different diseases.
The major factors that drive the market include the increasing or high burden of growth disorders and diabetes and technological advancements in the development of recombinant hormonal therapies. For instance, as per the IDF Diabetes Atlas 2021, the number of adults that are living with diabetes worldwide is estimated to be 537 million. This high burden of diabetes increases the need for advanced treatment using recombinant insulin and is expected to drive the market over the study period.
Furthermore, the new research studies on the recombinant hormones for accessing the safety profile increase the widespread of the products that help boost the market's growth. For instance, as per the study report published by Frontiers in Endocrinology in July 2022, PEGylated recombinant human growth hormone, Jintrolong, exhibited good long-term safety in cynomolgus monkeys and human pediatric growth hormone deficiency patients. The better safety profile of the product increases the usage of the drug and is expected to propel the market over the forecast period.
Hence, the factors like the high burden of diabetes and new research studies on recombinant hormones are expected to increase the usage of recombinant hormones and likely boost the market growth. However, adverse effects associated with recombinant hormonal therapies and stringent regulatory processes are the factors that hinder market growth over the forecast period.
Recombinant Hormone Market Trends
Insulin Segment is Expected to Hold a Major Share in the Market Over the Forecast Period
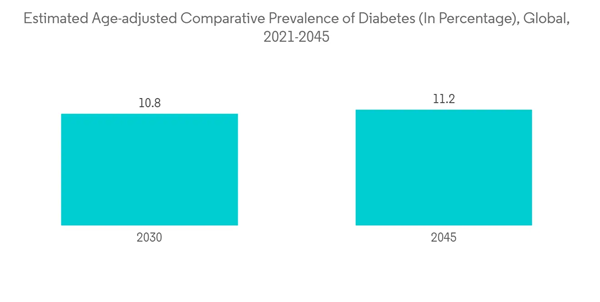
The insulin hormone is secreted by the beta cells of Islets of Langerhans of the pancreas, which catabolizes glucose in the blood. Insulin helps to keep blood sugar levels from getting too high (hyperglycemia) or too low (hypoglycemia). The increasing cases of diabetes, along with new developments and product launches, increase the usage of insulin, which is expected to boost the market over the forecast period.According to the IDF Diabetes Atlas 2021, the total number of adults with diabetes worldwide is estimated to be 643 million in 2030, which is expected to rise to 784 million by 2045. Hence, the increasing burden of diabetes is expected to have a significant impact on the recombinant insulin usage that drives the market over the forecast period.
Furthermore, new product developments and launches in the market segment are expected to boost the market's growth. For instance, in November 2021, Viatris Inc. and Biocon Biologics Ltd. launched the interchangeable biosimilars SEMGLEE (insulin glargine-yfgn), a recombinant human long-acting insulin analog, in the United States to help control high blood sugar in adult and pediatric patients with type 1 diabetes and adults with type 2 diabetes. Hence, the new product launches, along with the high burden of hormonal diseases, help to expand the product portfolio of the market players, which is expected to propel the market growth over the forecast period.
Thus, owing to the rising burden of diabetes and the launch of new products, the insulin segment of the market is expected to have considerable growth over the forecast period.
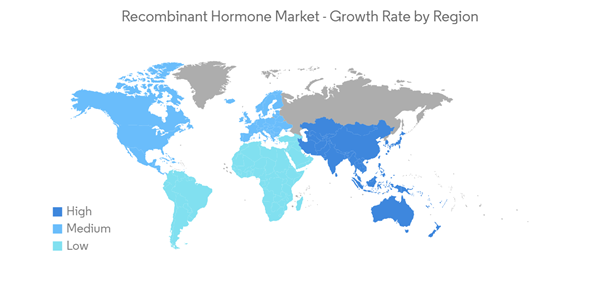
North America is Expected to Hold a Significant Share in the Market Over the Forecast Period.
North America is expected to hold a major market share in the recombinant hormone market owing to the increasing expenditure on research and increasing incidence of hormonal disorders. In addition to the above-mentioned factors, the presence of advanced healthcare infrastructure and several major market players in the region is expected to have significant growth in the market over the forecast period.According to the NIH RCDC 2022, the funding for the research on diabetes in the United States is estimated to be USD 1,124 million in 2021, which is expected to rise to USD 1,209 million by 2023. This research funding involves various categories, which also involve advanced treatment studies like recombinant hormones, and is expected to boost the market over the forecast period. Furthermore, new product developments and launches in the region increase the expansion of applications in the region.
For instance, in October 2021, Ascendis Pharma A/S launched SKYTROFA (lonapegsomatropin-tcgd), its once-weekly treatment for the treatment of pediatric patients one year and older who weigh at least 11.5 kg (25.4 lb) and have growth failure due to inadequate secretion of endogenous growth hormone (GH) in the United States. Similarly, in October 2021, Pfizer Canada ULC received approval for its next-generation long-acting growth hormone injection, PrNGENLA (somatrogon), from Health Canada. NGENLA is a once-weekly long-acting recombinant human growth hormone for the long-term treatment of pediatric patients who have growth failure due to an inadequate secretion of endogenous growth hormone (growth hormone deficiency or GHD). Thus, the new product launches in the region help increase the widespread applications, which are expected to boost the market over the forecast period.
Therefore, all the aforementioned factors, like the high prevalence of hormonal diseases and the latest company activities like product approvals and launches, are expected to increase the usage of recombinant hormone products and likely propel the market to grow over the forecast period.
Recombinant Hormone Industry Overview
The recombinant hormone market is consolidated and consists of a few major players. In terms of market share, a few of the major players are currently dominating the market. Collaborations and mergers over the next coming years to share technology and increasing investments for innovation in the field are expected to be observed. Some of the major market players include Eli Lilly and Company, Ferring Pharmaceuticals, Hoffmann La Roche Ltd (Genentech, Inc), Merck & Co Inc, Pfizer Inc, Novo Nordisk A/S, Novartis AG, Ipsen Pharma, LG Life Sciences and Teva Pharmaceutical Industries.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Eli Lilly and Company
- Ferring Pharmaceuticals
- F. Hoffmann-La Roche Ltd (Genentech, Inc)
- Merck & Co Inc
- Pfizer Inc
- Novo Nordisk A/S
- Novartis AG
- Ipsen Pharma
- LG Life Sciences
- Teva Pharmaceutical Industries
- Bio-Rad Laboratories, Inc
- Biocon