The emergence of COVID-19 led to a slight decline in the D-Dimer market during the initial phases of the pandemic, as there was a lack of availability of tests due to stringent lockdowns and production and manufacturing halts. But during the late stages, the pandemic had a significant impact on the market study as raising D-Dimer values can help identify people who can be at higher risk of COVID-19 complications, including disseminated intravascular coagulation and multi-organ failure. For instance, according to an article published in Dove Press in March 2022, coagulation disorders and acute respiratory failure were the prevailing causes of death from COVID-19. Together with other parameters that constitute the risk profile for severe COVID-19 evolution, the D-Dimers dosing at admission proved to be extremely useful in the management of COVID-19. Furthermore, during the post-pandemic phase, the market has seen a surge due to the rising adoption of D-Dimer, as this act as a biomarker for mortality in COVID-19 patients. For instance, according to another article published in Plos One, the D-Dimer value on admission was an accurate biomarker for predicting mortality in patients with COVID-19. This has led to the rising adoption of D-Dimer testing for the management of COVID-19 disease. Hence, the COVID-19 pandemic had a favorable impact on the market initially. Currently, as the pandemic has subsided, the market has lost some traction. However, it is expected to have stable growth during the forecast period.
A paradigm shift toward the demand for next-generation point-of-care D-Dimer testing has driven the market owing to the implementation of POC solutions significantly reducing waiting time, minimizing the length of patient stay, enabling faster diagnosis, and resulting in improved patient experience. The resounding benefits gained from these solutions in POC settings are considered to boost the growth of the market for D-Dimer testing. A rise in the incidence rate of Venous Thromboembolism (VTE), along with various pulmonary and cardiovascular diseases that cause life-threatening issues due to blood clot formation, is increasing the demand for D-Dimer testing.
According to a study published by the British Heart Foundation in August 2022, there were approximately 7.6 million people with heart and circulatory disease in the United Kingdom. Therefore, due to the high burden of cardiovascular diseases in developed countries such as the United Kingdom, there is an increasing demand for diagnostics assay, which can decrease the mortality rates associated with heart failure, thereby increasing the demand for D-Dimer assay during the study period.
According to an article published in the BMJ in February 2022, the diagnostic strategy using a combination of clinical pretest probability and D-Dimer identified patients at low risk for deep vein thrombosis during follow-up while substantially reducing the need for ultrasound imaging. Therefore, the importance of the D-Dimer test in identifying DVT helps in increased adoption, driving the market growth.
Therefore, owing to the aforementioned factors, the studied market is expected to grow significantly during the study period. However, the stringent regulation and limitations associated with the tests are expected to hinder the market growth during the study period.
Key Market Trends
The Pulmonary Embolism Segment is Expected to Witness a Robust Growth Over the Forecast Period
Pulmonary embolism is a blood coagulation that blocks and stops blood flow to an artery in the lung. The blood clot typically originates in a deep leg vein and goes to the lung.Growth of the pulmonary embolism segment is attributed to the growing global prevalence of this disease due to a rise in blood plasma D-Dimer levels among women and the rising geriatric population coupled with a higher burden of associated diseases.
The high prevalence of pulmonary embolism in developed countries is expected to drive segment growth during the forecast period. According to the CDC update in June 2022, pulmonary embolism affects up to 900,000 people annually in the United States. Such a huge incidence of pulmonary embolism (PE) is anticipated to drive market growth due to the rising adoption of D-Dimer testing for PE management.
According to another article published in JAMA Network in January 2021, among patients with chronic obstructive pulmonary disease admitted to the hospital with an acute worsening of respiratory symptoms, pulmonary embolism was detected in 5.9% of patients using a predefined diagnostic algorithm. Thus, the high prevalence of pulmonary embolism will fuel the market during the forecast years.
In addition, according to the study published in BMC Pulmonary Medicine in May 2021, in patients with Tuberculous pleural effusion (TPE), D-Dimer is an objective biomarker for predicting PE. Due to its outstanding sensitivity and specificity for PE, a D-Dimer may eliminate pointless radiological examinations. Such advantages of D-Dimer in predicting PE will therefore lead to increased adoption to reduce the harmful radiological examination, propelling the segment growth.
Therefore, the factors such as the versatile advantages of D-Dimer testing in pulmonary embolism and the high prevalence of PE are expected to drive the segment growth.
North America Holds a Significant Share in the Market and Expected to do Same in the Forecast Period
North America is expected to hold a significant share of the D-Dimer market throughout the forecast period. This is due to the rising prevalence of cardiopulmonary disorders and blood disorders such as venous thromboembolism, strokes, and disseminated intravascular coagulation among the huge population of elder individuals, generating higher demand for the D-Dimer test market.Furthermore, the market in this region is driven due to the factors such as the developed healthcare infrastructure in the United States, the strong foothold of key market players in the region, and rising healthcare expenditure, among others.
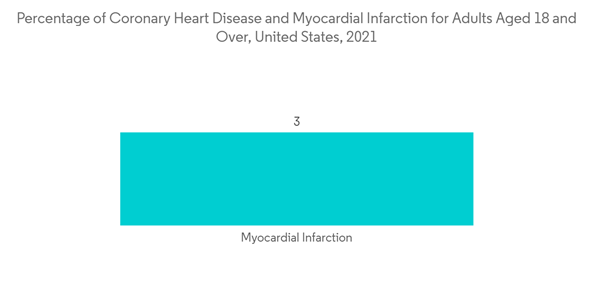
As per the CDC update in December 2022, the percentage of coronary heart disease and heart attack for adults aged 18 and over in the United States in 2021 was 4.9% and 3%, respectively. Such a huge prevalence of heart diseases in developed countries will lead to a rise in the adoption of D-Dimer testing for the diagnosis of coronary artery embolism, driving the market growth.
Furthermore, the University of Florida said D-Dimer testing is effective in individuals with COVID-19. This screening blood test, which was validated originally for seriously ill patients without COVID-19, is still useful to rule out pulmonary embolisms (PE) in hospitalized COVID-19 patients. Such research helps in the increased adoption of D-Dimer testing in the region, driving the market growth.
Therefore, factors such as the rising prevalence of cardiopulmonary diseases and rising initiatives from the key market players are expected to drive the market growth over the forecast period.
Competitive Landscape
The D-Dimer market is moderately competitive and consists of several major players. Few of the key players are entering into partnerships to develop novel methods and test kits, while others are increasing their sales force through partnering with distributors to increase their market position globally. Some of the companies which are currently operating in the market are F. Hoffmann-La Roche Ltd, Abbott Laboratories, Bio-Rad Laboratories Inc., Thermo Fisher Scientific, Danaher Corporation (Beckton Dickinson), BioMerieux, Sysmex Corporation, Henry Schein Inc., and Merck KGaA.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Bio-Rad Laboratories Inc.
- Danaher Corporation (Beckman Coulter)
- Beckton Dickinson
- BioMerieux
- F Hoffmann-La Roche Ltd
- Siemens Healthineers
- Sysmex Corporation
- Merck KGaA
- Thermo Fisher Scientific
- Henry Schein Inc.
- LumiraDx