Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
For instance, according to WHO, the number of people living with diabetes increased from 200 million in 1990 to 830 million in 2022. The rise has been significantly faster in low- and middle-income countries compared to high-income nations, highlighting a growing global health challenge and the need for targeted prevention and management strategies in developing regions.
Innovations such as single-use technologies, automation, and continuous bioprocessing are transforming production capabilities, enhancing efficiency, and reducing costs. The expanding pipeline of biologic drugs and the emergence of biosimilars are creating new opportunities within the market. Regulatory agencies are also adapting to support accelerated approvals and manufacturing flexibility. As scientific research continues to advance, the biomanufacturing market is poised to become increasingly essential in addressing global healthcare challenges, enabling faster development and distribution of next-generation therapies.
Key Market Drivers
Adoption of Advanced Technology and New Innovation
The rise in the geriatric population and the prevalence of chronic respiratory disorders are anticipated to drive the demand for ventilators. Increasing awareness of lung cancer symptoms and a growing number of patients in medical facilities contribute to the significant market growth of ventilators. For instance, in November 2023, the FDA approved Ixchiq, the first vaccine for chikungunya, for adults aged 18 and older. In December 2023, the FDA approved Casgevy and Lyfgenia - the first cell-based gene therapies - for treating sickle cell disease in patients aged 12 and older, marking major milestones in infectious disease prevention and genetic disorder treatment.However, it should be noted that the use of mechanical ventilation may pose certain risks, such as increased infection risk and potential damage to the lungs. These considerations should be taken into account when assessing the market growth potential of Bio-Manufacturing. Advanced technologies such as automation, robotics, and process control systems can streamline biomanufacturing processes, reduce human error, and enhance overall production efficiency. This can lead to quicker turnaround times and increased production capacity, meeting the growing demand for biologics.
Innovative bioreactor designs, single-use technologies, and flexible manufacturing platforms allow for easier scalability of production. As demand for bio manufactured products grows, the ability to quickly scale up production will become essential. Continuous manufacturing approaches, as opposed to traditional batch processes, can lead to consistent product quality, reduced wastage, and improved resource utilization. These advantages can boost demand for bio manufactured products.
Key Market Challenges
Huge Capital Expenditure
The biomanufacturing process involves intricate and specialized equipment, facilities, and technologies, which can require significant financial investments. Setting up a biomanufacturing facility or upgrading existing infrastructure requires substantial initial capital investment. This includes the construction or renovation of specialized cleanrooms, purchase of bioreactors, purification equipment, and other sophisticated tools necessary for biopharmaceutical production.The substantial capital required for biomanufacturing can divert resources away from other critical areas such as research and development, marketing, and business expansion. This resource allocation challenge can impact a company's overall growth strategy. The high capital expenditure can result in overestimation or underutilization of manufacturing capacity. If demand for the manufactured product is lower than anticipated, the investment may not yield the expected returns.
Key Market Trends
Emergence of Continuous Biomanufacturing
The emergence of continuous biomanufacturing has the potential to significantly boost the growth of the biomanufacturing industry in the future. Continuous biomanufacturing represents a departure from traditional batch processes by enabling the seamless, uninterrupted production of biopharmaceuticals and other biologically derived products. This innovative approach offers several benefits that can positively impact efficiency, flexibility, cost-effectiveness, and overall market expansion. Continuous biomanufacturing allows for continuous monitoring and adjustment of process parameters in real-time.This leads to improved process control, reduced variability, and enhanced product consistency, resulting in higher process efficiency and reduced production times. Continuous biomanufacturing systems are generally more compact and require less physical space than traditional batch systems. This reduction in facility footprint can lead to cost savings and greater flexibility in facility design and location. Continuous biomanufacturing can enable higher production capacities by running processes continuously, thereby increasing output without the need for significant facility expansion. This increased capacity can meet the growing demand for biopharmaceuticals and other biologically derived products.
Key Market Players
- Illumina Inc.
- Thermo Fischer Scientific Inc.
- Oxford Nanopore Technologies plc
- Agilent Technologies, Inc.
- BGI Genomics Co. Ltd.
- PerkinElmer Inc.
- QIAGEN NV
- Eurofins Scientific Inc.
- F. Hoffmann-La Roche Ltd
- Takara Bio Inc.
Report Scope:
In this report, the Global Bio-Manufacturing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Bio-Manufacturing Market, By Workflow:
- Continuous Upstream Biomanufacturing
- Single-Use Upstream Biomanufacturing
- Downstream Biomanufacturing
Bio-Manufacturing Market, By Application:
- Monoclonal Antibodies
- Hormones
- Vaccines
- Recombinant Proteins
- Others
Bio-Manufacturing Market, By End User:
- Biopharmaceutical Companies
- Research Institutions
- CMOs/CDMOs
Bio-Manufacturing Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Bio-Manufacturing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Illumina Inc.
- Thermo Fischer Scientific Inc.
- Oxford Nanopore Technologies plc
- Agilent Technologies, Inc.
- BGI Genomics Co. Ltd.
- PerkinElmer Inc.
- QIAGEN NV
- Eurofins Scientific Inc.
- F. Hoffmann-La Roche Ltd
- Takara Bio Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | August 2025 |
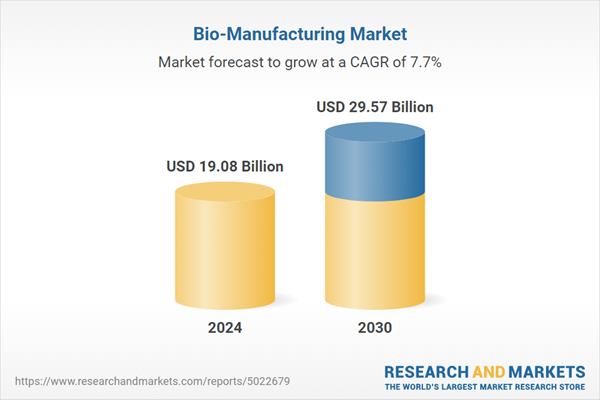
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 19.08 Billion |
| Forecasted Market Value ( USD | $ 29.57 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |