Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Automated external defibrillators (AEDs) are widely available in public places and can be used by untrained individuals in emergency situations to potentially save someone experiencing cardiac arrest. Other types of defibrillators, such as wearable cardioverter defibrillators (WCDs) and implanted cardioverter defibrillators (ICDs), are used to prevent sudden death in individuals at high risk of life-threatening arrhythmias. It may take time and effort to adjust to having a defibrillator at home, so it is important to be aware of any potential issues.
Key Market Drivers
Growing Incidence of Cardiovascular Diseases
Cardiac arrest remains a leading cause of mortality in many regions worldwide, despite advancements in emergency cardiac care that enhance a patient's chances of survival. Research indicates that approximately 350,000 Americans succumb to heart disease annually, with half of these fatalities occurring unexpectedly outside of medical facilities when the heart ceases to function. Sudden cardiac arrest, characterized by its abrupt onset, accounts for the majority of these untimely deaths. Ventricular fibrillation, an irregular heart rhythm disorder, is typically responsible for triggering sudden cardiac arrest.The automated external defibrillator (AED) is gaining popularity due to its ability to administer electric shocks for treating abnormal heart rhythms. These devices are designed to detect and treat two specific types of cardiac arrhythmias: pulseless ventricular tachycardia and ventricular fibrillation. In the coming years, the global market for AEDs is anticipated to present revenue-generating opportunities, driven by the increasing use of these devices in treating patients experiencing sudden cardiac arrest. Furthermore, the growing number of individuals dependent on smoking and alcohol consumption could contribute to prosperous market prospects.
Key Market Challenges
External Defibrillators Accessibility Issues
The American College of Cardiology has estimated that in the event of cardiac arrest, the likelihood of an automated external defibrillator (AED) being nearby is only 1 in 5. Additionally, there is a 20-30% chance of the AED being inaccessible because it may be inside a closed building. Out-of-hospital cardiac arrest in the U.S. results in over 356,000 fatalities annually, with a survival rate of less than 10-12%. Despite the proven benefits, the use of AEDs in public settings is not widespread due to concerns about legal liability, ambiguous training requirements, limited access, and lack of general awareness. However, research has unequivocally demonstrated that immediate access to an AED significantly enhances the chances of survival.Key Market Trends
Advancements in Next-Generation Defibrillators
The defibrillator market is projected to experience accelerated growth, surpassing initial expectations, owing to advancements in next-generation defibrillators. Defibrillators play a crucial role in identifying and resolving device-related issues. Sudden Cardiac Arrest (SCA), a leading cause of global mortality, carries potential fatality. However, early intervention and defibrillation can effectively manage this condition.Among external defibrillators, Automated External Defibrillators (AEDs) dominate the market and are expected to witness significant expansion, driven by the increasing number of heart failure patients relying on them. The growing demand aligns with the availability of a wider range of AEDs. Currently, wearables hold a competitive edge over manual external defibrillators. Furthermore, market growth is anticipated to receive a boost from Subcutaneous Implantable Cardioverter-Defibrillators (S-ICDs), MRI-compatible Implantable Cardioverter-Defibrillators (ICDs), and Cardiac Resynchronization Therapy Defibrillators (CRT-Ds) in the upcoming years.
Key Market Players
- Koninklijke Philips N.V.
- Stryker Corporation
- Zoll Medical Corporation
- Nihon Kohden Corporation
- ProgettiSrl
- Schiller AG
- MS Westfalia GmbH
- Bexen Cardio
- Silverline Meditech Pvt. Ltd.
- Mediana Co., Ltd.
Report Scope:
In this report, the Global Automated External Defibrillator Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Automated External Defibrillator Market, By Product Type:
- Manual External Defibrillator
- Fully Automated External Defibrillator
- Wearable Cardioverter Defibrillator
Automated External Defibrillator Market, By End User:
- Hospitals
- Pre-Hospitals
- Public Access Market
- Alternate Care Market
- Home
Automated External Defibrillator Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Automated External Defibrillator Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
Table of Contents
Companies Mentioned
- Koninklijke Philips N.V.
- Stryker Corporation
- Zoll Medical Corporation
- Nihon Kohden Corporation
- ProgettiSrl
- Schiller AG
- MS Westfalia GmbH
- Bexen Cardio
- Silverline Meditech Pvt. Ltd.
- Mediana Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | August 2025 |
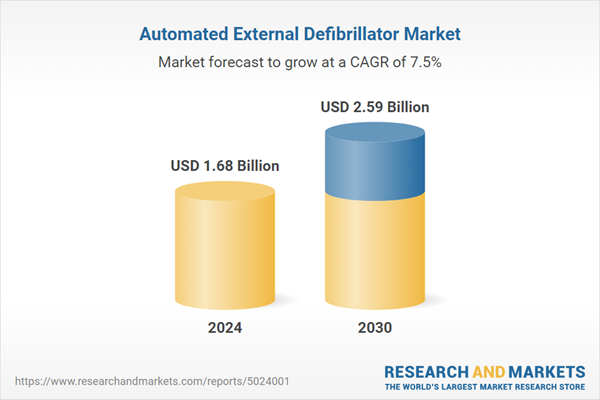
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.68 Billion |
| Forecasted Market Value ( USD | $ 2.59 Billion |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |