Global In-Vitro Colorectal Cancer Screening Tests Market - Key Trends and Drivers Summarized
What Are In-Vitro Colorectal Cancer Screening Tests and Why Are They Vital?
In-vitro colorectal cancer screening tests are diagnostic tools used to detect the presence of colorectal cancer or its precursors through non-invasive samples such as blood, stool, or other biological specimens. These tests are designed to identify specific biomarkers, such as DNA mutations, blood traces, or abnormal protein levels, which may indicate the presence of cancerous or pre-cancerous cells in the colon or rectum. As colorectal cancer remains one of the most common and deadly cancers worldwide, early detection is critical to improving patient outcomes. In-vitro screening offers a less invasive, more accessible alternative to traditional colonoscopy, which can often be intimidating or uncomfortable for patients. The ability of these tests to detect cancer at an early stage - when treatment is most effective - makes them a crucial tool in reducing mortality rates and improving long-term survival for patients at risk.How Are In-Vitro Colorectal Cancer Screening Tests Changing the Game in Early Detection?
In-vitro colorectal cancer screening tests are revolutionizing the approach to early cancer detection by providing easier, more patient-friendly methods of screening. Traditional methods, such as colonoscopy, although highly effective, have often faced resistance from patients due to the invasive nature of the procedure. In contrast, in-vitro tests, which involve analyzing blood or stool samples for signs of cancer, are much less invasive and can often be conducted in the comfort of a patient's home, enhancing compliance and screening rates. Tests like fecal immunochemical tests (FIT), stool DNA tests, and blood-based assays have simplified the screening process, making it more accessible to populations that are either unwilling or unable to undergo routine colonoscopies. These tests are especially significant for high-risk groups, including those with a family history of colorectal cancer or individuals over the age of 50, who require regular screening. By increasing the ease of screening, in-vitro tests are helping to identify cancer at its earliest stages, before symptoms appear. This not only improves the chances of successful treatment but also lowers the healthcare burden by reducing the need for more invasive procedures when the disease is caught early. With these advancements, patient care is becoming more preventive and less reactive, enabling medical professionals to address colorectal cancer more effectively and proactively.What Technological Breakthroughs Are Shaping In-Vitro Colorectal Cancer Screening?
The field of in-vitro colorectal cancer screening has seen significant advancements in technology, particularly in the areas of biomarker detection, molecular diagnostics, and automation. One major development is the use of next-generation sequencing (NGS) technology, which enables the detailed analysis of genetic mutations and DNA alterations associated with colorectal cancer. NGS allows for the detection of multiple genetic markers simultaneously, increasing the accuracy and reliability of screening tests. Additionally, liquid biopsy technologies, which analyze circulating tumor DNA (ctDNA) in the bloodstream, are emerging as a powerful tool for early cancer detection, offering a minimally invasive option for monitoring and screening. Artificial intelligence (AI) and machine learning are also playing a critical role in improving the sensitivity and specificity of in-vitro tests. By analyzing large datasets and identifying patterns in biomarker levels, AI-powered algorithms can enhance the precision of cancer detection, minimizing false positives and negatives. This helps in refining the screening process and ensures that patients receive timely and accurate diagnoses. Furthermore, advancements in automation are making these tests more efficient and scalable, reducing the cost and time required for analysis. This is particularly important as healthcare systems strive to make colorectal cancer screening more accessible and cost-effective for widespread use.What Is Driving the Rapid Growth of the In-Vitro Colorectal Cancer Screening Market?
The growth in the in-vitro colorectal cancer screening market is driven by several factors, including increasing awareness of early detection, advancements in screening technology, and the rising prevalence of colorectal cancer. As public health campaigns emphasize the importance of regular cancer screening, particularly for high-risk individuals, demand for non-invasive and accessible testing methods is surging. In-vitro tests, which provide a more patient-friendly alternative to traditional screening procedures like colonoscopy, are gaining popularity due to their convenience, lower cost, and reduced discomfort, encouraging more people to undergo regular screening. Technological advancements are another key driver. Innovations in molecular diagnostics, biomarker discovery, and liquid biopsy technologies are significantly enhancing the sensitivity and specificity of in-vitro tests, making them more accurate and reliable. This is expanding the market by attracting healthcare providers and patients who previously relied on traditional methods. Additionally, the aging global population, coupled with the increasing incidence of colorectal cancer, is fueling demand for effective early detection solutions. As more people fall into the high-risk age bracket for colorectal cancer, the need for accessible and non-invasive screening tools continues to grow. Moreover, regulatory support and expanding healthcare infrastructure in emerging markets are further contributing to the market's growth. Governments and healthcare organizations are increasingly endorsing in-vitro colorectal cancer screening as part of national screening programs, driving adoption across various regions. Together, these factors are propelling the in-vitro colorectal cancer screening market forward, helping to improve early detection rates and, ultimately, patient outcomes in the fight against colorectal cancer.Report Scope
The report analyzes the In-Vitro Colorectal Cancer Screening Tests market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Segment (Fecal Occult Blood Test, Biomarker Test, CRC DNA Screening Test); End-Use (Hospitals, Clinical Diagnostic Laboratories, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Fecal Occult Blood Test segment, which is expected to reach US$881.5 Million by 2030 with a CAGR of 3.8%. The Biomarker Test segment is also set to grow at 1.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $248.2 Million in 2024, and China, forecasted to grow at an impressive 5.9% CAGR to reach $233.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global In-Vitro Colorectal Cancer Screening Tests Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global In-Vitro Colorectal Cancer Screening Tests Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global In-Vitro Colorectal Cancer Screening Tests Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Inc., Alere, Inc., Beckman Coulter, Inc., Clinical Genomics Technologies Pty Ltd., Eiken Chemical Co., Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this In-Vitro Colorectal Cancer Screening Tests market report include:
- Abbott Laboratories, Inc.
- Alere, Inc.
- Beckman Coulter, Inc.
- Clinical Genomics Technologies Pty Ltd.
- Eiken Chemical Co., Ltd.
- Epigenomics AG
- Exact Sciences Corporation
- Hemosure, Inc.
- Novigenix SA
- Quidel Corporation
- Sysmex Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories, Inc.
- Alere, Inc.
- Beckman Coulter, Inc.
- Clinical Genomics Technologies Pty Ltd.
- Eiken Chemical Co., Ltd.
- Epigenomics AG
- Exact Sciences Corporation
- Hemosure, Inc.
- Novigenix SA
- Quidel Corporation
- Sysmex Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
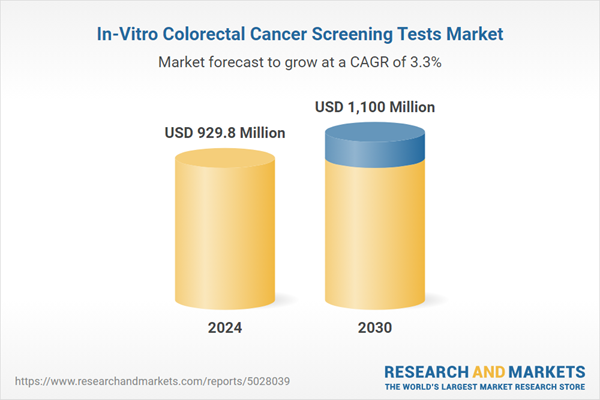
| Estimated Market Value ( USD | $ 929.8 Million |
| Forecasted Market Value ( USD | $ 1100 Million |
| Compound Annual Growth Rate | 3.3% |
| Regions Covered | Global |