Global Epinephrine Autoinjectors Market - Key Trends & Drivers Summarized
Why Are Epinephrine Autoinjectors Essential in Emergency Medicine?
Epinephrine autoinjectors are life-saving medical devices designed for the rapid administration of epinephrine (adrenaline) in cases of severe allergic reactions (anaphylaxis). These self-injecting devices provide a pre-measured dose of epinephrine, ensuring immediate relief by counteracting life-threatening symptoms such as airway constriction, swelling, and a dangerous drop in blood pressure. Given the increasing prevalence of severe allergies worldwide, epinephrine autoinjectors have become an essential tool in emergency medicine and personal healthcare.The convenience and ease of use of autoinjectors make them indispensable for patients with severe allergies, including those triggered by food allergens, insect stings, latex, and medications. Epinephrine autoinjectors are carried by individuals at risk of anaphylaxis, stored in schools, workplaces, and public institutions, and used by first responders to provide immediate intervention before emergency medical care arrives. As awareness of anaphylaxis grows, the demand for user-friendly, affordable, and accessible autoinjectors is increasing.
What Market Trends Are Driving Demand for Epinephrine Autoinjectors?
The global epinephrine autoinjector market is experiencing steady growth, driven by factors such as rising allergy prevalence, regulatory support for emergency medication access, and technological advancements in drug delivery systems. One of the most significant trends fueling demand is the increasing incidence of anaphylaxis. The global rise in food allergies, particularly among children, has led to heightened awareness and a greater need for autoinjectors as a preventive and emergency treatment measure.Another major factor is regulatory mandates requiring wider availability of epinephrine autoinjectors in schools, public places, and transportation systems. Many governments have introduced policies ensuring that schools and restaurants stock emergency epinephrine to respond to allergic reactions, further expanding the market. The growth of over-the-counter (OTC) autoinjectors and generic alternatives has also improved accessibility, making epinephrine treatment more affordable.
The rise of needle-free and compact autoinjector designs is transforming the market. Manufacturers are developing more ergonomic, easy-to-carry, and child-friendly devices that improve patient compliance. Innovations such as retractable needle designs, voice-guided instructions, and connected autoinjectors with smartphone integration have enhanced the usability and effectiveness of these devices.
Price reduction efforts and competition from generic brands have played a crucial role in market expansion. With the high cost of branded epinephrine autoinjectors sparking concerns over affordability, governments and healthcare organizations have encouraged the production of low-cost, generic alternatives to improve accessibility. This shift is making epinephrine autoinjectors more available to low-income and underserved populations, further increasing adoption.
Lastly, increased patient education and advocacy for anaphylaxis preparedness have raised awareness about the importance of carrying epinephrine autoinjectors. Medical professionals, allergy awareness groups, and social campaigns have emphasized the life-saving benefits of immediate epinephrine administration, encouraging more patients to keep autoinjectors on hand.
How Is Innovation Shaping the Epinephrine Autoinjector Market?
Innovation in epinephrine autoinjectors is focused on improving usability, safety, accessibility, and cost-effectiveness. One major area of advancement is the development of smaller, more portable devices. Traditional autoinjectors have been bulky, making them inconvenient for daily carry. New designs now emphasize compact and discreet formats, allowing patients to carry them comfortably in pockets, bags, and emergency kits.Needle-free epinephrine delivery systems are emerging as a significant innovation, particularly for needle-phobic patients. These devices replace traditional needles with jet injectors, using high-pressure technology to deliver epinephrine through the skin without puncturing it. This innovation reduces fear and anxiety associated with injections, improving patient compliance.
The integration of smart technology into autoinjectors is also gaining traction. Some new devices feature audio-visual guidance to help users administer the dose correctly, reducing errors in emergency situations. Additionally, Bluetooth-enabled autoinjectors can send alerts to caregivers or emergency services when activated, ensuring faster medical intervention.
Another key advancement is the development of multi-dose autoinjectors. Traditional autoinjectors are single-use, requiring patients to carry multiple units for safety. However, new autoinjectors with multi-dose capabilities allow for repeated use within a short period, particularly beneficial for individuals with severe allergies or those at risk of biphasic anaphylaxis, where symptoms reappear after initial treatment.
Regulatory advancements have also led to the rise of generic and biosimilar autoinjectors, making life-saving epinephrine more affordable. The introduction of auto-retracting needles and tamper-proof designs has improved safety and compliance, preventing accidental misuse or injury.
Furthermore, improvements in drug formulation are enabling extended shelf-life autoinjectors, reducing the frequency of replacements and making it more convenient for long-term use.
What Factors Are Driving Growth in the Epinephrine Autoinjector Market?
The global growth of the epinephrine autoinjector market is being driven by several factors, all of which highlight the increasing need for effective, accessible, and affordable emergency allergy treatment.One of the most significant drivers is the rising prevalence of severe allergies and anaphylaxis. The increasing incidence of food allergies, insect venom allergies, and drug-induced anaphylaxis has heightened the demand for readily available emergency treatment options. More patients and caregivers are recognizing the importance of carrying an epinephrine autoinjector, leading to higher prescription rates and increased market penetration.
Regulatory support and government initiatives have further expanded market growth. Many countries have implemented legislation requiring schools, restaurants, and workplaces to stock epinephrine autoinjectors for emergency use. Additionally, policies promoting insurance coverage for epinephrine prescriptions have made it easier for patients to obtain autoinjectors without financial barriers.
The availability of affordable generic alternatives has also contributed to market expansion. In the past, high prices and limited competition led to significant affordability issues for patients requiring epinephrine autoinjectors. However, the entry of multiple generic brands has lowered costs, increased supply, and improved accessibility for patients across various income levels.
The growth of direct-to-consumer and over-the-counter (OTC) sales has made epinephrine autoinjectors more widely available without requiring a prescription. This shift is particularly important in regions with limited healthcare access, ensuring that patients can quickly obtain life-saving medication when needed.
Rising public awareness and educational campaigns on anaphylaxis preparedness have led to greater adoption of epinephrine autoinjectors. Healthcare professionals, allergy organizations, and social media campaigns have encouraged at-risk individuals to keep an epinephrine autoinjector on hand, reinforcing the importance of early intervention during an allergic reaction.
Technological advancements in drug delivery have played a significant role in expanding the market. Innovations such as compact, portable designs, smart autoinjectors, and needle-free options are making epinephrine administration more convenient and accessible, encouraging more patients to carry and use these devices.
Finally, increasing demand from emerging markets has created new growth opportunities. As awareness, healthcare infrastructure, and regulatory approvals improve in developing regions, the demand for cost-effective epinephrine autoinjectors is expected to rise, driving further market expansion.
Report Scope
The report analyzes the Epinephrine Autoinjectors market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Dosage (0.15 gm, 0.30 gm, 0.5 gm); End-Use (Hospitals & Clinics, Individuals).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the 0.15 gm Dosage segment, which is expected to reach US$2.7 Billion by 2030 with a CAGR of 5.3%. The 0.30 gm Dosage segment is also set to grow at 6.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $807.1 Million in 2024, and China, forecasted to grow at an impressive 5.2% CAGR to reach $634.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Epinephrine Autoinjectors Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Epinephrine Autoinjectors Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Epinephrine Autoinjectors Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ALK-Abello A/S, Amneal Pharmaceuticals, Inc., Bausch + Lomb UK Ltd- Emerade, Certa Dose, Crossject SA and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Epinephrine Autoinjectors market report include:
- ALK-Abello A/S
- Amneal Pharmaceuticals, Inc.
- Bausch + Lomb UK Ltd- Emerade
- Certa Dose
- Crossject SA
- Halozyme Therapeutics, Inc.
- Kaleio, Inc.
- Pfizer, Inc.
- Teva Pharmaceutical Industries Ltd.
- Viatris, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ALK-Abello A/S
- Amneal Pharmaceuticals, Inc.
- Bausch + Lomb UK Ltd- Emerade
- Certa Dose
- Crossject SA
- Halozyme Therapeutics, Inc.
- Kaleio, Inc.
- Pfizer, Inc.
- Teva Pharmaceutical Industries Ltd.
- Viatris, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
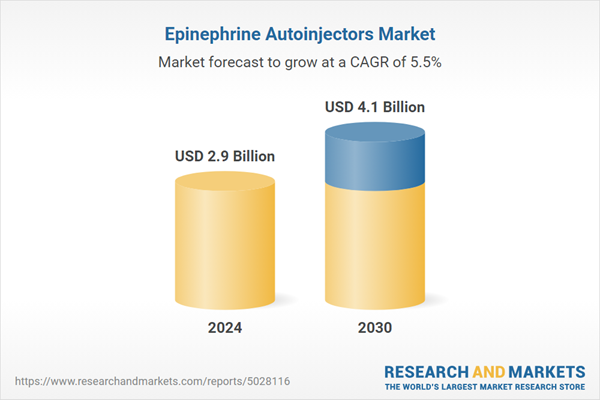
| Estimated Market Value ( USD | $ 2.9 Billion |
| Forecasted Market Value ( USD | $ 4.1 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |