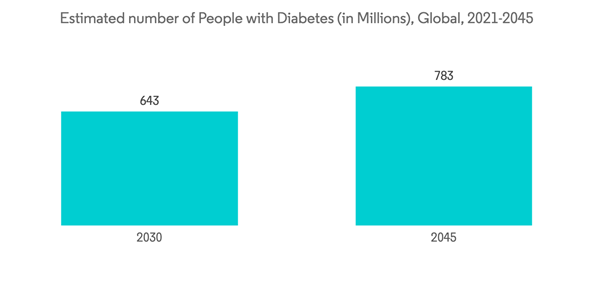The emergence of the COVID-19 pandemic had an adverse effect on the world economy and the healthcare system. The global lockdown has affected the supply chain of pharmaceuticals, medical devices, and biotechnological products. On the other hand, research and development have come into focus. All government agencies and healthcare players have come forward to support the development of diagnostics and treatment methods for COVID-19. As per the study "Retinal findings in patients with COVID-19: Results from the SERPICO-19 study," published in September 2020, COVID-19 could affect the retina. Retinal veins diameter seems directly correlated with the disease severity. Thus, during COVID-19, retinal diseases increased, impacting the demand for retinal biologics, thereby driving the market.
Certain factors driving the market growth include the rising burden of retinal diseases, the increasing diabetic patient population, increasing R&D activities, and the growing number of FDA approvals.
There has been a tremendous increase in the diabetic population, across the globe, over the past decade. Several reports and surveys documented a drastic increase in the diabetic population based on the changing lifestyle and habits. For instance, according to the Asian Diabetics Prevention Initiatives, in 2020, 60% of the diabetic population globally lived in Asia. The higher contribution of diabetes burden are China and India, which is 113.9 million and 65.1 million adults with diabetes in China and India, respectively, and by 2030, both China and India combined may have almost half a billion diabetic patients.
As per the study "Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis," published in November 2021, the number of adults globally with diabetic retinopathy was estimated to be 103.12 million in 2020, and by 2045, the numbers are projected to increase to 160.50 million. Thus, the growing burden of retinal diseases such as diabetic retinopathy may increase the demand for biologic treatment, which is expected to further drive the market growth.
Furthermore, in February 2022, Eyebiotech Limited secured USD 65 million in a series A financing round for developing a diverse pipeline of therapies for eye diseases such as wet age-related macular degeneration, diabetic retinopathy, etc. Therefore, rising investments may also boost innovation in the studied market, thereby driving the studied market growth.
However, the stringent regulatory process and initial high capital investment are the major restraining factors for the retinal biologics market.
Key Market Trends
Diabetic Retinopathy Shows Lucrative Opportunity in the Global Retinal Biologics Market
Diabetic retinopathy (DR) is a microvascular complication caused by high blood sugar due to diabetes. Too much sugar in the blood over time damages the retina. Factors such as growing diabetes, increasing research and development, and the launch of the product are driving the market segment's growth over time. According to the article published by International Diabetes Federation in March 2020, the devastating consequences of diabetes mellitus are set to continue due to the predicted increase in prevalence from 463 million in 2019 to 700 million in 2045. Among this diabetic population, the global prevalence of diabetic retinopathy (DR) and diabetic macular edema (DME) for 2019 was 27.0%. The lowest prevalence was in Europe at 20.6% and South East Asia at 12.5%, and the highest in Africa at 33.8%, the Middle-East and North Africa at 33.8%, and the Western Pacific region at 36.2%.The market segment growth is also contributed by the launch of products and approval from the global regulatory authorities. For Instance, in May 2019, Regeneron Pharmaceuticals, Inc. received United States Food and Drug Administration (FDA) approval for EYLEA (aflibercept) Injection to treat all stages of diabetic retinopathy. Such approvals are driving the growth of the market over the forecast period.
The most clinically important risk factors for progression to vision loss include the duration of diabetes, hyperglycemia, and hypertension. Control of serum glucose and blood pressure are effective in preventing vision loss due to DR. Hence, the rising prevalence of DR and the growing awareness and concern over it are expected to drive the segment growth over the forecast period.
North America Dominates the Global Retinal Biologics Market
The primary driving factors for the growth of the North American retinal biologics market are the rising burden of retinal diseases, the growing burden of the diabetic population in the region, the rise in research and development activities, increasing product launches, and rising strategic initiatives by key market players.The United States within North America is expected to hold a significant share of the studied market during the study period. For instance, as per the article published by International Diabetes Federation, in December 2021, 51 million adults aged between 20-79 years were living with diabetes in the North America and Caribbean Region in 2021. This number is estimated to increase to 57 million by 2030 and 63 million by 2045.
According to the data published by the Centers for Disease Control and Prevention (CDC), in 2020, around 4.1 million Americans were affected with diabetic retinopathy, and nearly 900,000 Americans were threatened with vision-damaging retinopathy. Diabetic retinopathy is one of the leading disorders of posterior segment eye disorders. Increasing prevalence may increase the demand for treatment in a huge population which may boost the market growth in the country.
Moreover, strategic initiatives taken by the key market players, such as product launches, partnerships, initiation of new programs, and mergers and acquisitions, may drive market growth. For instance, in October 2021, Novartis received the United States Food and Drug Administration (FDA) for the company’s supplemental Biologics License Application (sBLA) for the type-II variation application for Beovu (brolucizumab) 6 mg for the treatment of diabetic macular edema (DME). In July 2021, Genentech, a member of the Roche Group, announced that the US Food and Drug Administration (FDA) accepted the company’s Biologics License Application (BLA) for faricimab for the treatment of wet, or neovascular, age-related macular degeneration (AMD), diabetic macular edema (DME) and diabetic retinopathy.
Competitive Landscape
The retinal biologics market is competitive and consists of a few major players. The strategies such as mergers and acquisitions adopted by the key market player may boost the market growth. Companies like F. Hoffmann-La Roche Ltd, Regeneron Pharmaceuticals Inc., AbbVie Inc., MeiraGTx Limited, and Oxurion NV, among others, hold a substantial market share in the market.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- F. Hoffmann-La Roche Ltd
- Regeneron Pharmaceuticals Inc.
- AbbVie Inc.
- MeiraGTx Limited
- Oxurion NV
- Novartis
- GenSight Biologics
- Adverum Biotechnologies
- SemaThera Inc.
- Amgen