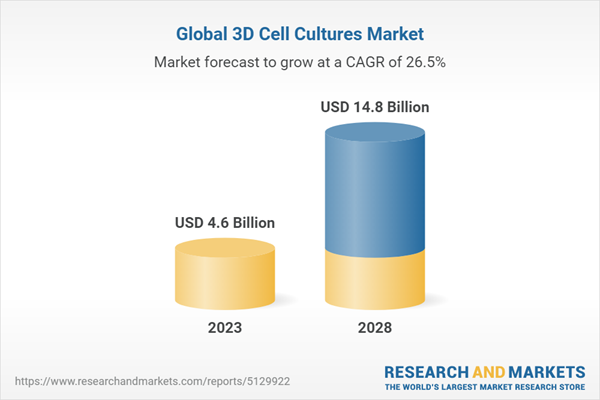
Chapter 2 Summary and Highlights
- Market Outlook
- Market Summary
Chapter 3 Market Overview
- An Opening Comment on an Amazing Industry
- Industry Issues
- In Vitro versus In Vivo
- Dimensionality
- The Research Chain for 2D and 3D Cell Culture
- Best Practices
- Standardization
- Regulation
- Genomics Forcing the Hand of the FDA
- Leachables and Extractables
- Broad Issues
- Research Talent Shortages
- The Shifting International Picture
- Pace and Diversification of Innovation
- A Comment on the "Other" Areas of Cell Culture
- Omics Everywhere
- Is 2020 a Watershed Year for the Cell Culture Industry?
- Preliminary Market Analysis
- Cell Culture Market Growth Rate Estimates
- Assessing Large-Scale Media Consumption Needs
- Modeling Future Growth in Biopharmaceuticals
- Base Case for the Cell Culture Market
- Challenges in Projecting Sales and Growth
- Cell Culture Media Market Estimates
- Cell and Gene Therapy Bioprocessing Segment
- Evaluating Media Consumption for Biosimilars
- What About CDMOs?
- Microfluidics
- Bioreactors
- Internal Cell Culture Resources
- Is There Too Much Concentration of Ownership in Biotechnology?
- Characterizing Innovation in 3D Cell Culture
- Bioprinting Strategic Roadmap
Chapter 4 Market, by Type
- Where Did Tissue and Cell Culture Start?
- History and Early Applications
- Invention of Tissue Culture
- Development of Sustained Cell Lines
- First Cell Culture Flask and Rigorous Techniques
- Lindbergh: The Cell Culture Equipment Pioneer
- Establishing Continuous Cell Lines
- Key Developments in Equipment
- Terminology and Concepts
- Tissue and Cell Culture Industry
- Tissue Culture and Cell Culture Definitions
- Cell Lines
- Care and Growth of Cell Culture Systems
- Media, Sera, and Reagents
- Gels and Scaffolds
- Microplates/Microtiter Plates
- Bioanalytical Instruments
- Bioanalytical Imaging
- Bioprinting
- Bioreactors
- Other Equipment for Cell Culture
- Adherent Approaches
- Traditional Roller Bottles
- Other Systems
- Information Technology: Software and Services for the Cell Culture Research Market
- Software for the Research Market in Cell Culture
- Software-Related Support Services
- Bioprocessing Consumables for Cell Culture
- Microcarriers for Large-Scale 3D Culture
- Sera for Large-Scale 3D Culture
- Media for Large-Scale 3D Culture
- Bioreactor Bags for Large-Scale 3D Culture
- Other
- Bioprocessing Equipment
- Analytical Equipment for Bioprocessing
- Automation Systems for Bioprocessing
- Support Equipment for Bioprocessing
- Aspects of Large-Scale Manufacturing of Biopharmaceuticals and Vaccines
- Suspension Proteins and Monoclonal Antibodies
- Adherent-Cell-Based Therapies and Vaccines
- Small-Scale Adherent to Make Somatic Cells, Stem Cells and Tissues
- Vaccines
- Vaccine Development as a Catalyst
- Vaccines Developed Using Human Cell Strains
- Exosome Manufacturing
- Viral Vector Manufacturing
- Lentivirus Manufacturing
- Plasmid Manufacturing
- Cell Culture End Users
- Pharma/Biopharma
- Universities
- Government
- CROs/CDMOS
- Other
- Cell Culture Applications
- Drug Discovery
- Clinical Development
- Toxicology
- Basic Research
- Bioprocessing Development
- Other
- Regional Markets
- The Americas
- Europe
- Asia-Pacific
- Rest of the World
Chapter 5 Assays, Imaging and Analysis
- Assays
- Assay Development for Mesenchymal Stem Cells
- In Vitro Testing of Adventitious Agents
- Assays and Assay Kits
- Cell-Based Assays: Overview and Newer Developments
- Cells Used in Cell-Based Assays
- Notes on 3D Cell-Based Assays
- Kinetic Metabolism Assays
- Cell Proliferation
- Viability and Cytotoxicity
- Permeability Assays for Cell Viability and Survival
- Cell Invasion
- Cell Signaling and Communication
- Cytostatic
- Cell Death Assays
- Imaging Technology
- Imaging Assays
- Fluorescence as a Driver of Screening
- Analytical Systems Used in Tissue and Cell Culture
- Understanding "Cellomics"
- HCS Support of 3D Cell Culture
- NGS Discovery Pools
- Multiplex Assays
- Predictive Toxicology
- Neuro Safety
- The Omics Invasion
- Transcriptomics
Chapter 6 Regulation and Standardization
- U.S. Regulatory Status of Bioprinted Products
- Basic Guidance for the Regulation of Biologics
- Guidance for Regenerative Medicine
- Guidance for Xenotransplants
- Guidance for Regenerative Medicine: Emergency Approval
- Regulating Bioprinted Products
Chapter 7 3D Models for Cancer
- Disease Modeling
- Cancer
- Main Classes of Models for Researching Cancer and Other Diseases
- Cell Lines
- Spheroids and Organoids
- Genetically Engineered Mouse Model (GEMM)
- Patient-Derived Tumor Xenografts (PDXs)
- Overview: Cancer at the Cellular Level
- In Vivo (Animal) Testing Standard
- Empire of the Mouse
- Humanized Mice
- 2D Culture
- 3D Requirements
- Cell Number and Viability
- Migration and Invasion
- Unmet Needs: Angiogenesis and Immune System Evasion
- Benefits of 3D Models to Cancer Research
- Greater Distinction in Cell Morphology and Proliferation
- Greater Gene Expression and Cell Behavior
- Better Models of Cell Migration and Invasion
- Cell Heterogeneity
- Breast Cancer as a Driver of 3D Cultures
- Structure, Polarity and Apoptosis
- Melanoma as a Driver of 3D Cultures
- Moving to Spheroid Configurations
- 3D Systems in Cancer Research
- Multicellular Tumor Spheroids
- Multilayered Cell Cultures
- 3D Engineered Scaffolds
- Natural Materials
- Synthetic Materials
- Human Cancer Model Initiative (HCMI)
- Next-Generation Human Cancer Models
- Drug Sensitivity and Resistance
- Altered Signaling and Sensitivity
- Drug Resistance
- Cellular Signaling
- Cellular Signaling Mediated by Integrins
- Drug Screening
- Approaches and Endpoints
- Spheroid Applications
- Metastasis via 3D Cell Migration Model
- Metastasis via Lung-on-Chip
- Cancer Metabolism
- Future Horizons
- Metastases
- Co-culture
- Vascularization
- Cancer-Associated Fibroblasts
- Cancer Stem Cells
- Combination Therapies
- Biologics Development
- Tumor Recurrence
- Patient-Derived Cells
- Patient-Derived Tumor Xenografts (PDXs)
- Evolution of PDX Platforms
Chapter 8 Landscape for Toxicology and Drug Safety Testing
- Introduction
- Liver
- Toxicology Background
- Testing for Adverse Effects on the Skin
- New Assessment Methodologies Impact on 3D Cell Culture
- Toxicology Testing in Cosmetics
- Updated Regulatory Requirements
- Efficacy of Cosmetics and Cosmeceuticals
- Aspects of Cosmetic Toxicity Testing
- Skin Irritation
- Skin Corrosion
- Phototoxicity
- Skin Sensitization
- Eye Irritation
- Acute Systemic Toxicity
- Acute Toxicity Testing
- Cytotoxicity Assays for Acute Toxicity Testing
- Chronic and Repeated Dose Toxicity
- Carcinogenicity and Genotoxicity
- Overview
- In Vitro Methods: Background and Recent Developments
- Regulatory versus Drug Development Applications
- Efforts to Reduce False Positives
- Recent Innovations in Screening
- Future Challenge: Non-genotoxic Carcinogens
- Reproductive and Developmental Toxicity
- Background
- Following the Reproductive Cycle
- Development and Reproductive Tox Testing Types
- Zebrafish Model for Developmental Toxicity Screening
- Combination of Zebrafish and Stem Cells
- Biomedical Frontiers: Male Testis
- Endocrine Disruptor Screening
- Background
- Environmental Toxicology Impacts In Vitro Methods
- ToxCast and Tox 21 Initiatives
- Future Challenge: Thyroid Disruption
- BG1 Assay
- Toxicokinetics and ADME
- In Vitro Developments
- Metabolism
- Pharmacokinetics of Low-Turnover Compounds
- Organotypic Models
- 3D Models for Skin
- 3D Corneal System
- Absorption Barrier Models
- Gastrointestinal
- Lung
- Blood-Brain Barrier
- Real Architecture for 3D Tissue Barriers and Extracellular Matrix
- Liver Toxicity
- Uniqueness and Complexity of Liver
- Liver as a Key Driver for 3D Innovation
- In Vitro Liver Applications
- In Vivo Liver Function and Structure
- Liver Metabolism
- In Vitro Liver Models
- Co-cultures of Hepatocytes and Macrophages
- 3D Liver Models
- Bioprinted Liver Tissue
- Detected Hepatosphere Structures and Functionality
- Ideal Criteria
- Drug Resistance
- Transporter Studies
- Achieving Heterotypic Cell-Cell Contacts
- Unmet Needs and Future Drivers of Innovation
- Morphogen Signaling
- Multi-donor Liver Cells
- Kidney Toxicity Applications
- Future Challenge: Stem-Cell Derived Kidney Cells
- Bioprinted Kidney Tissue
- Pancreatic Toxicology Applications
- Cardiovascular Toxicity
- Commercial Avenue
- Collaboration with Regulators
- Cardiovascular Drug Discovery
- Microelectrode Arrays (MEAs) Based on Impedance
- Surrogate for Aortic Ring Assay via Bioprinted Magnetics
- Vasodilator Activity
- 3D Engineered Heart Tissues
Chapter 9 Market Breakdown by Region
- Introduction
- North America
- United States
- Canada
- Europe
- Germany
- United Kingdom
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Rest of the World
Chapter 10 Stem Cell Landscape
- A History of Stem Cells
- Major and Minor Research Areas for Stem Cells
- 3D Stem Cell Culture Systems
- Plate or Culture Dish
- Spinner Flask and Rotating Wall Vessel
- Perfusion Bioreactor and Microcarrier Systems
- Shortcomings
- Microfluidics and Stem Cells
- Short Review of Stem Cell Biology
- Embryogenesis
- Growth, Structure and Morphology of Stem Cells
- Stem Cell Differentiation
- Stem Cell Differentiation versus Proliferation
- Extracellular Matrix and Stem Cells
- Soluble Factors
- Manufacturing Stem Cells
- Controlling Embryoid Body Formation
- Forced Aggregation Cultures
- Hanging-Drop Approach
- Applications
- Stem Cell Markers for High-Throughput Screening
- Teratomas and the Teratoma Assay
- Fujifilm (Cellular Dynamics Inc.)
- Stem Cell Research Breakthroughs
- Stem Cells for Neuroscience Discovery and Development
- Example: Alzheimer's Research
- Background: B-Amyloid Cascade Hypothesis
- Human iPSC-Derived Models
- New 3D Model
- Other Advantages of 3D
- Envisioned Applications
- Other 3D Neuro Applications
- Stem Cells for Cardiovascular Discovery
- Stem Cells for the Development of Regenerative Medicine
- Background: Allogenic versus Autologous
- MicroRNAs
- Induced Pluripotent Stem Cells (IPS)
Chapter 11 Regenerative Medicine: Organ Transplants and Skin Substitutes
- Regenerative Medicine
- Need for Organ Transplants
- Applications in Regenerative Medicine
- Investments in Regenerative Medicine
- Skin Substitutes Industry
- Tissue Culture Allograft and Autograft Products
- Tissue Engineering in Regenerative Medicine
Chapter 12 Company Profiles
- 3D BIOPRINTING SOLUTIONS
- 3D BIOTEK LLC
- 4D TECHNOLOGY CORP.
- ABCAM PLC
- AKRON BIOTECH
- AGILENT TECHNOLOGIES INC.
- ALPCO
- AMSBIO
- BECKMAN COULTER INC.
- BIOINSPIRED SOLUTIONS
- BIOTIME INC.
- BIOVISION INC.
- CELL APPLICATIONS INC.
- CELLINK
- CORNING INC.
- CYPROTEX
- CYTIVA
- CYTOO SA
- EMD MILLIPORE / MERCK KGAA
- EMULATE INC.
- ENVISIONTEC INC.
- EPITHELIX
- EUROFINS SAS
- GREINER BIO-ONE INTERNATIONAL GMBH
- HAMILTON ROBOTICS
- HUB ORGANOIDS
- HUREL CORP.
- INSPHERO
- INVITROCUE
- KIYATEC INC.
- LIFENET HEALTH
- LOREM VASCULAR / CYTORI THERAPEUTICS INC.
- MATTEK
- MIMETAS INC.
- ORGANOVO HOLDINGS INC.
- PERKINELMER INC.
- PLASTICELL LTD.
- PLURISTEM THERAPEUTICS INC.
- POIETIS
- PROMEGA CORP.
- SEAHORSE BIOSCIENCE
- STEMCELL TECHNOLOGIES
- STRATATECH CORP.
- SYNVIVO INC.
- TAP BIOSYSTEMS
- TECAN TRADING AG
- ZEN-BIO INC.
List of Tables
Summary Table: Global Market for 3D Cell Culture, by Segment, Through 2028
Table 1: Dimensionality of Cell Culture
Table 2: WHO R&D Roadmap of Priority Infectious Diseases
Table 3: 3D Bioprinting Roadmap
Table 4: Selected Online Prices for Bioreactors
Table 5: Global Market for 3D Cell Culture, by Segment, Through 2028
Table 6: Tissue Types
Table 7: Cell Types Based on Developmental Origin
Table 8: Leading Cell Line Suppliers, April 2020
Table 9: Commonly Used Transformed Cells Lines
Table 10: Major Primary Cell Lines
Table 11: Leading Primary Cell Suppliers
Table 12: Main Types of Stem Cells
Table 13: Stem Cell Services
Table 14: Areas of Interest in 3D Spheroid Research
Table 15: Selected Nanoparticle Products Used in Life Science Research
Table 16: Results of Liver-Chip Drugs Halted in Previous Clinical Trials Based on Animal Studies
Table 17: Companies and Universities Involved in the Organ-on-a-Chip Industry
Table 18: Microfluidics Companies
Table 19: Selected Recent Patents Issued on Microfluidic Devices Related to Cell Culture Applications
Table 20: Selected Papers Published on Recent Microfluidic Advances in Cell Culture
Table 21: High-Content Screening Suppliers and Key Attributes
Table 22: Selected Patents Issued Related to Flow Cytometers
Table 23: Thermo Fisher Imaging Products
Table 24: Recent Bioprinting Company Deals and Strategic Partnerships
Table 25: Bioprinting Modalities
Table 26: Maintaining Cell Viability During Printing
Table 27: Bioprinting Instrument Industry
Table 28: Biomaterial Components
Table 29: Bioink Types
Table 30: Classes of Matrix Bioink Hydrogels
Table 31: Matrix Bioink Selection Criteria
Table 32: Selected Bioink Companies, 2020
Table 33: Projected Unit Sales of Research Bioreactors, < 10 Liters, 2020
Table 34: Leading Bioreactor Suppliers, 2020
Table 35: Label-Free Technologies and Suppliers
Table 36: Developmental Issues Facing the Commercialization of Exosomes
Table 37: Companies Working on Exosome Products
Table 38: Global Market for Cell Culture, by End User, Through 2028
Table 39: Global Market for Cell Culture, by Application, Through 2028
Table 40: Global Market for Cell Culture, by Region, Through 2028
Table 41: Typical Assay Endpoints and Tests
Table 42: U.S. Patents on Assays Systems, 2019 and 2020
Table 43: Selected U.S. Patents on Assay Imaging, 2019 and 2020
Table 44: Recently Issued U.S. Patents on Cellomics, 2019
Table 45: Toxicology Issues That Need to Be Addressed for FDA-Regulated Products
Table 46: Overview of Federal Regulation of the Cell Culture Markets
Table 47: FDA List of Cell, Biologic and Tissue Products Regulated Under CBER and CDRH
Table 48: FDA “Talking Point” Recommendations for Regenerative Medicine Advanced Therapies (RMATs)
Table 49: U.S. Regulatory Considerations for Bioprinted Biologics
Table 50: Main Types of Models for Researching Cancer and Other Diseases
Table 51: Goals of Funding Opportunity Announcement RFA-CA-19-055
Table 52: Common 3D Assays
Table 53: Summary of the Benefits and Advantages of EV3D
Table 54: ECVAM List of Current Activities, 2020
Table 55: In Vitro Testing in Cosmetics, by Test Class
Table 56: Global Market for Cell Culture by Region, Through 2028
Table 57: North American Market for Cell Culture by Country, Through 2028
Table 58: European Market for Cell Culture by Country, Through 2028
Table 59: Asia-Pacific Market for Cell Culture by Country, Through 2028
Table 60: RoW Market for Cell Culture by Region, Through 2028
Table 61: Major and Minor Research Areas in Stem Cells
Table 62: Stem Cell Usage in Research
Table 63: Recent U.S. Patents Granted on Stem Cell Technologies
Table 64: Papers Published on Selected Stem Cell Research Trends
Table 65: Comparison of the Number of Citations in the Literature on Cell Culture versus Stem Cell Culture
Table 66: Number of Organ Transplants Performed in the United States, 2018 and 2019
Table 67: Total Global Financing of Regenerative Medicine
Table 68: Total Financing of Regenerative Medicine, by Therapeutic Area
Table 69: Leading Tissue Products and Suppliers
Table 70: Printed Tissue and Organs: Commercialization Timeframe
Table 71: Tissue/Organ Complexity
Table 72: 3D Bioprinting Solutions: Company Snapshot
Table 73: 3D Biotek LLC: Company Snapshot
Table 74: 4D Technology Corp.: Company Snapshot
Table 75: Abcam Plc: Company Snapshot
Table 76: Abcam PLC: Financial Performance, 2022
Table 77: Akron Biotech: Company Snapshot
Table 78: Agilent Technologies: Company Snapshot
Table 79: Agilent Technologies Inc.: Financial Performance, 2022
Table 80: Alpco: Company Snapshot
Table 81: Amsbio: Company Snapshot
Table 82: Beckman Coulter: Company Snapshot
Table 83: Bioinspired Solutions: Company Snapshot
Table 84: BioTime Inc.: Company Snapshot
Table 85: Biovision Inc.: Company Snapshot
Table 86: Cell Applications Inc.: Company Snapshot
Table 87: Cellink: Company Snapshot
Table 88: Corning Inc.: Company Snapshot
Table 89: Corning Inc.: Financial Performance, 2022
Table 90: Cyprotex: Company Snapshot
Table 91: Cytiva: Company Snapshot
Table 92: Cytoo SA: Company Snapshot
Table 93: Merck KGAA: Company Snapshot
Table 94: Merck KGAA: Financial Performance, 2022
Table 95: Emulate Inc.: Company Snapshot
Table 96: EnvisionTec Inc.: Company Snapshot
Table 97: Epithelix: Company Snapshot
Table 98: Greiner Bio-One International GmbH: Company Snapshot
Table 99: Hamilton Robotics: Company Snapshot
Table 100: HUB Organoids: Company Snapshot
Table 101: Hurel Corp.: Company Snapshot
Table 102: Insphero: Company Snapshot
Table 103: Invitrocue: Company Snapshot
Table 104: Kiyatec Inc.: Company Snapshot
Table 105: LifeNet Health: Company Snapshot
Table 106: Cytori Therapeutics Inc.: Company Snapshot
Table 107: Mattek: Company Snapshot
Table 108: Mimetas Inc.: Company Snapshot
Table 109: Organovo Holdings Inc.: Company Snapshot
Table 110: PerkinElmer Inc.: Company Snapshot
Table 111: Plasticell Ltd.: Company Snapshot
Table 112: Pluristem Therapeutics Inc.: Company Snapshot
Table 113: Poietis: Company Snapshot
Table 114: Promega Corp.: Company Snapshot
Table 115: SeaHorse Bioscience: Company Snapshot
Table 116: Stemcell Technologies: Company Snapshot
Table 117: Stratatech Corp.: Company Snapshot
Table 118: Synvivo Inc.: Company Snapshot
Table 119: Tap Biosystems: Company Snapshot
Table 120: Tecan Trading AG: Company Snapshot
Table 121: Zen-Bio Inc.: Company Snapshot
List of Figures
Summary Figure: Global Market for 3D Cell Culture, by Segment, 2020-2028
Figure 1: Research Chain for Cell Culture
Figure 2: A Model for The Evolution of FDA Regulation
Figure 3: 3D Cell Cultures: Market Dynamics
Figure 4: An Innovation Matrix
Figure 5: Bioprinting Strategic Roadmap
Figure 6: Global Market Shares of 3D Cell Culture, by Segment, 2022
Figure 7: Gastrointestinal Organotype Cultures
Figure 8: iCELLis Nanoreactor: Example of Commercial 2D Cell Culture Systems
Figure 9: Prototype of 3D Model Lung-on-a-Chip from Wake Forest
Figure 10: Recent Photo of the HepaChip
Figure 11: Recent Photo of the HepaChip-MWP
Figure 12: University of Toronto Handheld Bioprinting Device
Figure 13: Collaborative Experiment Conducted by American, Russian and Israeli Scientists
Figure 14: Smart Marbles Concept for Quantifying Process Heterogeneity
Figure 15: Porcine Intestinal Organoids
Figure 16: Diagram of the Components of a Predictive Toxicology System
Figure 17: Gleason’s Pattern
Figure 18: Diagram of PDXs, Cell Lines and Organoid/Spheroid Xenografts
Figure 19: Cell Heterogeneity and Its Function
Figure 20: Global Market for Cell Culture by Region, 2020-2028
Figure 21: North American Market for Cell Culture by Country, 2020-2028
Figure 22: European Market for Cell Culture by Country, 2020-2028
Figure 23: Asia-Pacific Market for Cell Culture by Country, 2020-2028
Figure 24: ROW Market for Cell Culture by Region, 2020-2028
Figure 25: Electron Micrograph of Porous Microcarrier for Stem Cell Production
Figure 26: Photograph of Apligraf
Figure 27: Abcam PLC: Financial Performance, 2021 and 2022
Figure 28: Abcam PLC: Revenue Shares, by Country/Region, 2022
Figure 29: Agilent Technologies Inc.: Financial Performance, 2021 and 2022
Figure 30: Agilent Technologies Inc.: Revenue Shares, by Business Unit, 2022
Figure 31: Agilent Technologies Inc.: Revenue Shares, by Country/Region, 2022
Figure 32: Corning Inc.: Revenue Shares, by Business Unit, FY 2022
Figure 33: Corning Inc.: Revenue Shares, by Region, FY 2022
Figure 34: Merck KGAA: Revenue Shares, by Business Unit, FY 2022
Figure 35: Merck KGAA: Revenue Shares, by Region, FY 2022