Global Enzyme-linked Immunosorbent Assay (Elisa) Testing Market - Key Trends and Drivers Summarized
How Is Enzyme-Linked Immunosorbent Assay (ELISA) Testing Revolutionizing Diagnostics and Research?
Enzyme-Linked Immunosorbent Assay (ELISA) testing is revolutionizing diagnostics and research by providing a highly sensitive and specific method for detecting and quantifying proteins, hormones, antibodies, and other biomolecules. Widely used in clinical laboratories, pharmaceutical research, and biotechnology, ELISA tests are essential for diagnosing infectious diseases, monitoring immune responses, and detecting biomarkers related to various health conditions, including cancer, HIV, and autoimmune disorders. The ability to measure specific antigens or antibodies with precision makes ELISA an indispensable tool for both healthcare providers and researchers. The technique's versatility allows it to be applied in disease diagnostics, vaccine development, drug efficacy testing, and food safety monitoring. As the demand for accurate, reliable, and cost-effective diagnostic methods grows, ELISA testing continues to play a critical role in advancing public health, medical research, and therapeutic development, providing the foundation for timely and effective interventions.What Innovations Are Enhancing the Functionality of ELISA Testing?
Innovations in automation, multiplexing, and point-of-care (POC) testing are significantly enhancing the functionality of ELISA tests. Automation has transformed ELISA workflows by allowing high-throughput screening, reducing human error, and increasing the speed of analysis. Automated ELISA systems, which include robotic pipetting, automated plate readers, and integrated software, enable laboratories to process hundreds or thousands of samples simultaneously, improving efficiency and reducing turnaround time for diagnostic results. These systems also standardize procedures, ensuring consistent results across different tests and reducing variability that can occur with manual processes. Automation is particularly important in large-scale clinical diagnostics, pharmaceutical research, and epidemiological studies where the rapid processing of samples is essential for timely decision-making.Multiplexing, which allows multiple analytes to be measured in a single sample, is another major advancement in ELISA technology. Traditional ELISA tests typically measure one target at a time, but multiplex ELISA kits can detect and quantify several biomarkers simultaneously. This innovation significantly reduces the time and resources needed to analyze complex biological samples, such as blood or serum, providing comprehensive data in a single assay. Multiplex ELISA is particularly useful in research settings where multiple cytokines, hormones, or disease markers need to be measured to understand disease mechanisms or treatment efficacy. This capability is also beneficial in personalized medicine, where simultaneous measurement of multiple biomarkers helps tailor treatments to individual patients.
Point-of-care (POC) ELISA testing represents another breakthrough, making it possible to perform ELISA tests outside of traditional laboratories, such as in clinics, remote areas, or even at home. Portable ELISA devices are designed to provide rapid, on-site diagnostic results, often within minutes. These systems are particularly valuable in low-resource settings or during emergencies, where quick diagnosis and treatment are critical. The development of POC ELISA tests for diseases like HIV, hepatitis, and COVID-19 has greatly expanded access to diagnostics, improving healthcare delivery in underserved populations. These portable systems are often user-friendly, with simplified workflows that can be operated by non-specialists, broadening the reach of ELISA technology.
Nanotechnology is also enhancing the sensitivity of ELISA tests. Nanomaterials, such as gold nanoparticles, are being integrated into ELISA assays to amplify signals and improve detection limits, making it possible to detect extremely low concentrations of biomarkers. This is particularly important in early disease detection, where identifying low levels of a specific antigen or antibody can lead to earlier interventions and better patient outcomes. These advancements in sensitivity are helping to push the boundaries of ELISA testing, allowing for more accurate diagnosis and research at the molecular level.
How Does ELISA Testing Impact Disease Diagnosis and Treatment?
ELISA testing has a profound impact on disease diagnosis and treatment by providing a reliable, sensitive, and accessible method for detecting disease-related biomarkers in blood, saliva, urine, or other biological samples. One of the primary benefits of ELISA is its ability to detect antibodies, antigens, and other proteins associated with infectious diseases, making it a crucial tool for diagnosing conditions like HIV, hepatitis, Lyme disease, and COVID-19. In HIV diagnostics, for example, ELISA is often used as an initial screening test to detect the presence of antibodies against the virus. Early detection of such infections is critical for initiating treatment, preventing disease progression, and reducing transmission.In oncology, ELISA tests are widely used to measure tumor markers, such as PSA (prostate-specific antigen) or CA-125 (cancer antigen 125), which help in diagnosing and monitoring cancer progression. By quantifying these markers, clinicians can assess tumor activity, evaluate the effectiveness of treatments, and detect recurrences. The high specificity of ELISA tests ensures that these markers can be accurately measured even in complex biological samples, providing essential information for personalized treatment planning. Similarly, in autoimmune diseases like lupus or rheumatoid arthritis, ELISA is used to detect the presence of specific autoantibodies, helping clinicians diagnose and manage these conditions more effectively.
In addition to diagnostics, ELISA testing plays a critical role in monitoring the immune response to vaccines and therapies. For example, in vaccine research, ELISA can measure the production of antibodies in response to vaccination, providing insights into vaccine efficacy and duration of protection. This has been particularly important during the development of COVID-19 vaccines, where ELISA tests were used to assess immune responses in clinical trials. ELISA is also used in therapeutic drug monitoring, where it helps clinicians ensure that patients are receiving optimal doses of biologics or other therapies by measuring drug levels in the blood.
ELISA's role in guiding treatment decisions extends beyond human healthcare. In veterinary medicine, ELISA tests are used to detect diseases in animals, such as bovine tuberculosis or avian influenza, helping to protect animal health and prevent the spread of zoonotic diseases to humans. Additionally, ELISA is widely employed in food safety testing to detect allergens, pathogens, and contaminants, ensuring that food products meet safety standards and are free from harmful substances.
By providing accurate, timely, and cost-effective diagnostics, ELISA testing significantly improves patient care, enhances disease management, and supports better outcomes across a range of medical and scientific applications.
What Trends Are Driving Growth in the ELISA Testing Market?
Several trends are driving growth in the ELISA testing market, including the increasing prevalence of infectious diseases, the rising demand for early cancer detection, the growth of personalized medicine, and advancements in automation and multiplexing technologies. The growing global burden of infectious diseases, such as HIV, hepatitis, and more recently, COVID-19, is one of the most significant factors fueling demand for ELISA tests. As healthcare systems focus on controlling the spread of these diseases, ELISA is a key tool for large-scale diagnostic screening, particularly in high-prevalence regions. The need for rapid, accurate, and accessible diagnostic tests in response to pandemic threats has further accelerated the adoption of ELISA testing in both clinical and point-of-care settings.The rising demand for early cancer detection is another major trend driving the ELISA market. As cancer rates continue to increase globally, there is a growing emphasis on developing diagnostic tools that can detect cancer at its earliest stages, when treatment is most effective. ELISA's ability to measure tumor markers and other cancer-related proteins makes it an essential part of early detection strategies, particularly in screening programs for prostate, ovarian, and breast cancer. The integration of ELISA with other diagnostic technologies, such as liquid biopsies and molecular diagnostics, is expected to enhance its role in oncology, offering more comprehensive and accurate cancer detection solutions.
The growth of personalized medicine is also contributing to the expansion of the ELISA testing market. Personalized medicine relies on the identification of biomarkers to tailor treatments to individual patients, and ELISA is a key tool for measuring these biomarkers. In conditions like autoimmune diseases, cardiovascular disorders, and cancers, personalized treatment plans depend on precise biomarker data, which ELISA provides. As the field of precision medicine grows, demand for ELISA testing to support biomarker discovery, drug development, and personalized treatment monitoring is expected to rise.
Advancements in automation and multiplexing technologies are further driving growth by making ELISA testing more efficient and scalable. Automated ELISA systems are reducing labor costs, minimizing human error, and enabling high-throughput testing, which is crucial for large-scale clinical diagnostics and research. Multiplex ELISA, which allows multiple biomarkers to be measured in a single assay, is streamlining workflows and providing more comprehensive data from each sample. These innovations are making ELISA more appealing for laboratories seeking to increase testing capacity while maintaining accuracy and consistency.
Regulatory and industry standards are also contributing to the growth of the ELISA market. As regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), enforce stricter guidelines on drug safety, vaccine efficacy, and diagnostic accuracy, ELISA testing is becoming increasingly important for ensuring compliance. Additionally, the rise of global health initiatives focused on disease control and prevention, such as the World Health Organization's (WHO) efforts to combat infectious diseases, is increasing the demand for reliable ELISA-based diagnostics.
These trends highlight the growing importance of ELISA testing in diagnostics, research, and personalized medicine. As technological advancements continue to improve the accuracy, speed, and accessibility of ELISA tests, the market is poised for sustained growth, driven by the need for precise, reliable, and scalable diagnostic solutions in an increasingly complex healthcare landscape.
Report Scope
The report analyzes the Enzyme-linked Immunosorbent Assay (Elisa) Testing market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Sandwich ELISA, Indirect ELISA, Multiple & Portable ELISA, Other Types); End-Use (Hospitals & Diagnostic Centers, Research Laboratories, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Sandwich ELISA segment, which is expected to reach US$784.1 Million by 2030 with a CAGR of 8%. The Indirect ELISA segment is also set to grow at 6.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $288.8 Million in 2024, and China, forecasted to grow at an impressive 7.2% CAGR to reach $248 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Enzyme-linked Immunosorbent Assay (Elisa) Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Enzyme-linked Immunosorbent Assay (Elisa) Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Enzyme-linked Immunosorbent Assay (Elisa) Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abcam PLC, American Laboratory Products Company (ALPCO), Apollo Diagnostics, Assay Biotechnology Company, Inc., Astra Biotech GmbH and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 22 companies featured in this Enzyme-linked Immunosorbent Assay (ELISA) Testing market report include:
- Abcam PLC
- American Laboratory Products Company (ALPCO)
- Apollo Diagnostics
- Assay Biotechnology Company, Inc.
- Astra Biotech GmbH
- Avioq, Inc.
- Awareness Technology, Inc.
- Axispharm Laboratories Ltd.
- Biogenes
- bioMerieux SA
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abcam PLC
- American Laboratory Products Company (ALPCO)
- Apollo Diagnostics
- Assay Biotechnology Company, Inc.
- Astra Biotech GmbH
- Avioq, Inc.
- Awareness Technology, Inc.
- Axispharm Laboratories Ltd.
- Biogenes
- bioMerieux SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 163 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
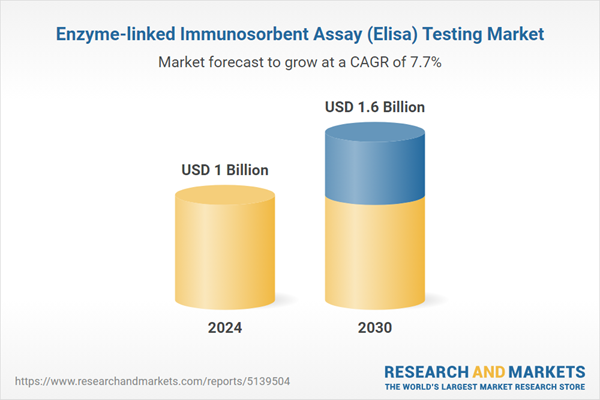
| Estimated Market Value ( USD | $ 1 Billion |
| Forecasted Market Value ( USD | $ 1.6 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |