Global CAR T-cell Therapy Market - Key Trends and Drivers Summarized
What Makes CAR T-Cell Therapy a Breakthrough in Cancer Treatment?
Chimeric Antigen Receptor (CAR) T-cell therapy has revolutionized the landscape of cancer treatment, particularly for patients battling certain types of blood cancers that have not responded to conventional therapies like chemotherapy and radiation. This form of immunotherapy works by harnessing and enhancing the body's own immune system to target and destroy cancer cells. The process begins with the extraction of a patient's T-cells, a type of white blood cell crucial to immune response. These T-cells are then genetically modified in a laboratory to express a receptor (CAR) that allows them to recognize specific proteins on the surface of cancer cells. Once the T-cells are modified and multiplied, they are reinfused into the patient's body, where they seek out and destroy the cancer cells with extraordinary precision. CAR T-cell therapy has shown remarkable efficacy, particularly in treating hematologic cancers such as B-cell lymphomas and acute lymphoblastic leukemia (ALL). In many cases, patients who had exhausted all other treatment options have experienced long-term remission. This therapy represents a significant leap forward in personalized medicine, as it is tailored to the individual's specific cancer profile, offering a customized approach that has been transformative in oncology.How Are Technological Innovations Enhancing the Efficacy of CAR T-Cell Therapy?
The development of CAR T-cell therapy has been significantly advanced by cutting-edge technologies that have improved both the efficacy of the treatment and its safety profile. One of the most critical innovations has been the design of more sophisticated CAR constructs, allowing for greater precision in targeting cancer cells. Early iterations of CAR T-cell therapy targeted a single antigen on cancer cells, but newer versions are engineered to recognize multiple antigens, reducing the chances of the cancer evading detection. This has been especially important in addressing challenges like antigen escape, where cancer cells mutate to avoid immune destruction. In addition, advancements in gene-editing technologies, such as CRISPR, have enabled scientists to modify T-cells with increased accuracy, enhancing their ability to target cancer cells while reducing the risk of unintended effects on healthy tissues. Technological improvements in cell manufacturing processes have also reduced the time needed to modify and expand T-cells, making the therapy more accessible and reducing wait times for patients with aggressive cancers. Furthermore, the integration of artificial intelligence (AI) and machine learning into the development and administration of CAR T-cell therapy has enabled researchers and clinicians to predict patient responses more accurately, optimizing the treatment process. These innovations not only enhance the effectiveness of CAR T-cell therapy but also contribute to its scalability, ensuring that this powerful treatment is available to a broader range of patients worldwide.What Are the Current Trends and Challenges in CAR T-Cell Therapy?
Several important trends are shaping the future of CAR T-cell therapy, reflecting both its promise and the challenges that must be addressed to maximize its potential. One of the most exciting trends is the expansion of CAR T-cell therapy beyond blood cancers to solid tumors. Historically, treating solid tumors has been more complex due to the protective environment these tumors create around themselves, making it difficult for immune cells to penetrate and attack. However, research is actively exploring ways to enhance T-cell infiltration into solid tumors and sustain their activity in the hostile tumor microenvironment. Another significant trend is the development of allogeneic, or 'off-the-shelf,' CAR T-cell therapies. Unlike the traditional approach that requires extracting and modifying a patient's own cells (autologous therapy), allogeneic CAR T-cell therapy uses T-cells from healthy donors. This approach promises to significantly reduce the time and cost associated with the therapy, potentially making it more widely accessible. Despite these advancements, several challenges remain. Managing severe side effects, such as cytokine release syndrome (CRS) and neurotoxicity, remains a critical concern. These side effects occur when the immune system is over-activated by the therapy, causing inflammation and potentially dangerous complications. Research is focused on mitigating these risks, and several safety mechanisms, including built-in 'suicide switches' that deactivate the CAR T-cells in emergencies, are being developed. Additionally, the high cost of manufacturing CAR T-cell therapy, often exceeding several hundred thousand dollars per patient, is a major barrier to broader adoption. This cost reflects the complexity of producing a personalized, living cell therapy, but efforts to streamline production and develop more efficient manufacturing techniques are underway to reduce costs.What's Driving Growth in the CAR T-Cell Therapy Market?
The growth in the CAR T-cell therapy market is driven by several factors, including technological advancements, expanding clinical indications, and the increasing demand for personalized cancer treatments. One of the primary drivers is the expanding body of clinical evidence demonstrating the efficacy of CAR T-cell therapy in treating various types of cancer, especially hematologic malignancies such as lymphoma, leukemia, and multiple myeloma. The potential for CAR T-cell therapy to move into the treatment of solid tumors represents another key growth opportunity, as ongoing research continues to investigate new therapeutic targets and applications in cancers like lung, breast, and pancreatic cancer. Technological improvements in gene-editing tools and cell manufacturing processes are also contributing to the market's growth, allowing for faster and more cost-effective production of CAR T-cells. The increasing demand for personalized medicine is another crucial factor driving market expansion, as patients and healthcare providers look for treatments tailored to the unique genetic and molecular profile of individual cancers. This is especially important in cases where traditional therapies have failed, making CAR T-cell therapy a vital option for those with few alternatives. Additionally, the global rise in cancer prevalence is fueling demand for more innovative treatments, particularly in regions where regulatory approvals for CAR T-cell therapies are expanding, such as in Europe and Asia. The development of allogeneic CAR T-cell therapies, which could provide more affordable and widely available treatment options, further accelerates market growth. With continuous advancements in AI, which are improving treatment precision and monitoring, and the growing focus on enhancing patient outcomes, the CAR T-cell therapy market is positioned for robust growth in the years to come.Report Scope
The report analyzes the CAR T-cell Therapy market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Indication (DLBCL, ALL, MM, CLL, FL).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the DLBCL Indication segment, which is expected to reach US$5.1 Billion by 2030 with a CAGR of 18.6%. The ALL Indication segment is also set to grow at 25.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $944 Million in 2024, and China, forecasted to grow at an impressive 21.4% CAGR to reach $1.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global CAR T-cell Therapy Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global CAR T-cell Therapy Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global CAR T-cell Therapy Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as A2 Biotherapeutics, Inc., Adaptive Biotechnologies Corporation, Advanced Cell & Gene Therapy, AffyImmune Therapeutics, Allogene Therapeutics and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 12 companies featured in this CAR T-cell Therapy market report include:
- A2 Biotherapeutics, Inc.
- Adaptive Biotechnologies Corporation
- Advanced Cell & Gene Therapy
- AffyImmune Therapeutics
- Allogene Therapeutics
- Beam Therapeutics
- CARsgen Therapeutics
- Celgene Corporation
- Cellular Biomedicine Group, Inc.
- Innovacell
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- A2 Biotherapeutics, Inc.
- Adaptive Biotechnologies Corporation
- Advanced Cell & Gene Therapy
- AffyImmune Therapeutics
- Allogene Therapeutics
- Beam Therapeutics
- CARsgen Therapeutics
- Celgene Corporation
- Cellular Biomedicine Group, Inc.
- Innovacell
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 179 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
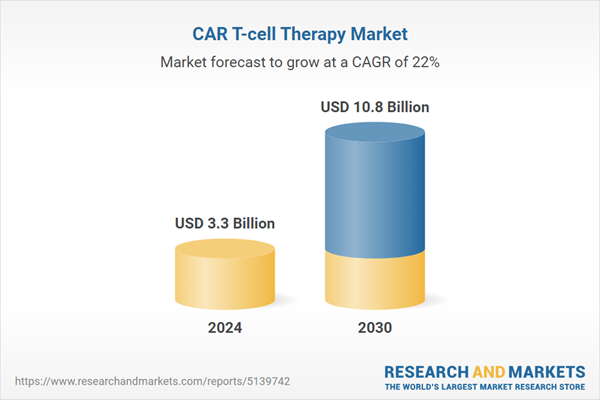
| Estimated Market Value ( USD | $ 3.3 Billion |
| Forecasted Market Value ( USD | $ 10.8 Billion |
| Compound Annual Growth Rate | 22.0% |
| Regions Covered | Global |