Global Interventional Neurology Devices Market - Key Trends & Drivers Summarized
What Are Interventional Neurology Devices and Why Are They Crucial in Treating Neurovascular Disorders?
Interventional neurology devices are specialized medical instruments used in the minimally invasive treatment of neurovascular disorders, such as strokes, aneurysms, and arteriovenous malformations (AVMs). These devices include catheters, stents, embolization coils, and clot retrieval systems, which are designed to navigate the intricate and delicate vasculature of the brain. Interventional neurology has become a crucial field in modern medicine, as it offers patients less invasive alternatives to traditional open surgeries, leading to faster recovery times, reduced risk of complications, and improved outcomes. As the prevalence of neurovascular disorders continues to rise globally, the demand for advanced interventional neurology devices is increasing, making them essential tools in the treatment and management of these life-threatening conditions.How Are Technological Advancements Shaping the Interventional Neurology Devices Market?
Technological advancements are significantly shaping the interventional neurology devices market, particularly through innovations in imaging, catheter design, and materials science. Advances in imaging technologies, such as high-resolution 3D angiography and real-time intraoperative imaging, are enhancing the precision and safety of neurovascular procedures by providing detailed visualization of the brain's vasculature. The development of more flexible and steerable catheters is improving the ability of interventionalists to navigate complex arterial pathways, reducing the risk of vessel injury and improving procedural success rates. Innovations in materials science, including the use of biocompatible and radiopaque materials, are enhancing the safety and efficacy of devices such as stents and embolization coils, making them more effective in treating complex neurovascular conditions. These technological trends are driving the adoption of interventional neurology devices across healthcare settings, as they enable more effective and less invasive treatment options for patients with neurovascular disorders.Why Is There an Increasing Demand for Interventional Neurology Devices in Healthcare?
The demand for interventional neurology devices is increasing in healthcare due to the growing prevalence of neurovascular disorders, the aging population, and the rising adoption of minimally invasive procedures. Stroke remains a leading cause of death and disability worldwide, driving the need for effective treatment options such as thrombectomy devices and embolization coils. The aging population is also contributing to the demand for interventional neurology devices, as older adults are at higher risk for conditions such as aneurysms and AVMs that require advanced interventional treatment. The trend towards minimally invasive procedures is further fueling demand, as patients and healthcare providers seek alternatives to open surgeries that offer faster recovery times, reduced hospital stays, and lower complication rates. Additionally, the increasing availability of specialized training for interventional neurologists and the expansion of stroke centers are enhancing the adoption of these devices, as more healthcare facilities are equipped to perform complex neurovascular procedures. As the burden of neurovascular diseases continues to grow, the demand for interventional neurology devices is expected to increase.What Factors Are Driving the Growth in the Interventional Neurology Devices Market?
The growth in the interventional neurology devices market is driven by several factors related to technological advancements, demographic trends, and the increasing focus on minimally invasive treatments. One of the primary drivers is the rising prevalence of neurovascular disorders, such as strokes and aneurysms, which require advanced interventional devices for effective treatment. The aging global population is also a significant growth factor, as older adults are more susceptible to neurovascular conditions that necessitate interventional care. The growing preference for minimally invasive procedures is further propelling the market, as these techniques offer numerous benefits over traditional open surgeries, including faster recovery and reduced risk of complications. Technological advancements in imaging, catheter design, and materials are also driving market growth by improving the safety, efficacy, and accessibility of interventional neurology procedures. Additionally, the increasing investment in healthcare infrastructure and the expansion of specialized stroke centers are creating new opportunities for the adoption of interventional neurology devices. As these factors continue to influence the healthcare landscape, the interventional neurology devices market is expected to experience sustained growth, driven by the need for advanced, effective, and minimally invasive treatment options for neurovascular disorders.Report Scope
The report analyzes the Interventional Neurology Devices market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Device (Cerebral Embolization & Aneurysm Coiling, Cerebral Angioplasty & Stenting Systems, Neurothrombectomy, Support); Therapeutic Area (Cerebral Aneurysm, Strokes, Cerebral Artery Stenosis, Other Therapeutic Areas).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Cerebral Embolization & Aneurysm Coiling Devices segment, which is expected to reach US$2 Billion by 2030 with a CAGR of 4.8%. The Cerebral Angioplasty & Stenting Systems segment is also set to grow at 3.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1 Billion in 2024, and China, forecasted to grow at an impressive 4.3% CAGR to reach $773.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Interventional Neurology Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Interventional Neurology Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Interventional Neurology Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, ACANDIS GmbH, B. Braun Melsungen AG, Bayer AG, Biosensors International Group Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Interventional Neurology Devices market report include:
- Abbott Laboratories
- ACANDIS GmbH
- B. Braun Melsungen AG
- Bayer AG
- Biosensors International Group Ltd.
- Boston Scientific Corporation
- DePuy Synthes
- evonos GmbH & Co. KG
- Medtronic, Inc.
- Merit Medical Systems, Inc.
- MicroPort Scientific Corporation
- Penumbra, Inc.
- Stryker Corporation
- Surtex Instruments
- Terumo Corporation
- W. L. Gore & Associates, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- ACANDIS GmbH
- B. Braun Melsungen AG
- Bayer AG
- Biosensors International Group Ltd.
- Boston Scientific Corporation
- DePuy Synthes
- evonos GmbH & Co. KG
- Medtronic, Inc.
- Merit Medical Systems, Inc.
- MicroPort Scientific Corporation
- Penumbra, Inc.
- Stryker Corporation
- Surtex Instruments
- Terumo Corporation
- W. L. Gore & Associates, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
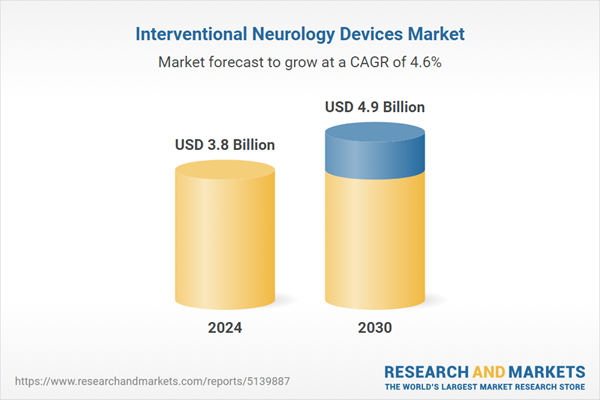
| Estimated Market Value ( USD | $ 3.8 Billion |
| Forecasted Market Value ( USD | $ 4.9 Billion |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |