Global Pediatric Interventional Cardiology Devices Market - Key Trends & Drivers Summarized
How Are Technological Advancements Revolutionizing Pediatric Interventional Cardiology Devices?
The pediatric interventional cardiology devices market has seen significant technological advancements, leading to improved outcomes in the treatment of congenital heart diseases (CHD) in children. These devices are designed to diagnose and treat heart conditions in pediatric patients using minimally invasive techniques, which have become increasingly favored over traditional open-heart surgeries due to their reduced recovery times and lower risk of complications. Innovations such as bioresorbable stents, advanced balloon catheters, and next-generation closure devices have made it possible to perform intricate heart repairs with greater precision. Imaging technologies such as 3D echocardiography and advanced cardiac MRI systems are being integrated into pediatric interventions, allowing for better visualization and planning of complex procedures. Additionally, the miniaturization of devices has enabled more tailored interventions for younger children and infants, ensuring that these devices can be used effectively in smaller anatomies. These advancements not only improve the safety and effectiveness of procedures but also open the door to new treatment possibilities for pediatric patients with complex heart conditions.Why Is the Demand for Pediatric Interventional Cardiology Devices Growing?
The demand for pediatric interventional cardiology devices is on the rise due to the increasing prevalence of congenital heart disease (CHD) worldwide. CHD remains the most common birth defect, affecting nearly 1% of live births globally, with many cases requiring medical intervention early in life. As diagnostic capabilities improve and more CHD cases are identified and treated earlier, the need for specialized pediatric cardiology devices continues to grow. Additionally, advancements in neonatal care and the ability to successfully treat very young and preterm infants have expanded the patient population requiring interventional cardiology procedures. Pediatric interventional cardiology devices provide minimally invasive treatment options, which are particularly important for this young and fragile patient population. The growing awareness of CHD and the push for earlier detection through improved screening methods are also contributing to increased demand. Furthermore, healthcare systems worldwide are recognizing the need for specialized pediatric cardiac care, prompting investments in pediatric cardiology programs and driving the market for interventional devices designed specifically for children.How Are Patient Care Trends and Regulatory Approvals Shaping the Pediatric Cardiology Market?
Trends in patient-centered care and stringent regulatory requirements are shaping the pediatric interventional cardiology devices market. With the rise of personalized medicine, there is a growing focus on developing devices tailored to the unique needs of pediatric patients, particularly in terms of size, flexibility, and biocompatibility. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are putting greater emphasis on ensuring that devices intended for pediatric use meet strict safety and efficacy standards. This has led to an increase in clinical trials focused on pediatric applications, ensuring that devices are specifically tested for use in children rather than being adapted from adult devices. Additionally, the trend toward value-based healthcare is driving demand for devices that not only improve patient outcomes but also reduce long-term costs by minimizing the need for repeat interventions. As healthcare providers continue to focus on patient outcomes and the quality of life post-intervention, the demand for cutting-edge pediatric interventional cardiology devices that meet these criteria is increasing.What Factors Are Driving Growth in the Pediatric Interventional Cardiology Devices Market?
The growth in the pediatric interventional cardiology devices market is driven by several factors, including technological innovations, the rising prevalence of congenital heart defects, and regulatory advancements. One key driver is the increasing use of minimally invasive techniques, which reduce the risks and recovery times associated with traditional surgeries, making these devices more attractive for treating children. Technological advancements, such as the development of bioresorbable stents and improved catheter systems, are enabling more effective and safer interventions. Additionally, the growing focus on early diagnosis of congenital heart diseases is boosting demand for these devices, as more children are being treated at younger ages. The rising investment in pediatric cardiology programs and research, along with government initiatives to improve neonatal and pediatric care, is further driving market expansion. Lastly, the increasing adoption of imaging technologies that improve the precision and outcomes of pediatric cardiac procedures is playing a significant role in the growth of this market, as healthcare providers continue to seek out advanced solutions that improve patient care.Report Scope
The report analyzes the Pediatric Interventional Cardiology Devices market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Segment (Congenital Heart Defect Closure Devices, Transcatheter Heart Valves, Other Segments).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Congenital Heart Defect Closure Devices segment, which is expected to reach US$1.4 Billion by 2030 with a CAGR of 4.5%. The Transcatheter Heart Valves segment is also set to grow at 4.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $617.6 Million in 2024, and China, forecasted to grow at an impressive 7.9% CAGR to reach $691.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pediatric Interventional Cardiology Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pediatric Interventional Cardiology Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pediatric Interventional Cardiology Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Boston Scientific Corporation, Cardinal Health, Inc., Edwards Lifesciences Corporation, GE Healthcare, Medtronic PLC and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Pediatric Interventional Cardiology Devices market report include:
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Edwards Lifesciences Corporation
- GE Healthcare
- Medtronic PLC
- Siemens AG
- W. L. Gore & Associates, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Edwards Lifesciences Corporation
- GE Healthcare
- Medtronic PLC
- Siemens AG
- W. L. Gore & Associates, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
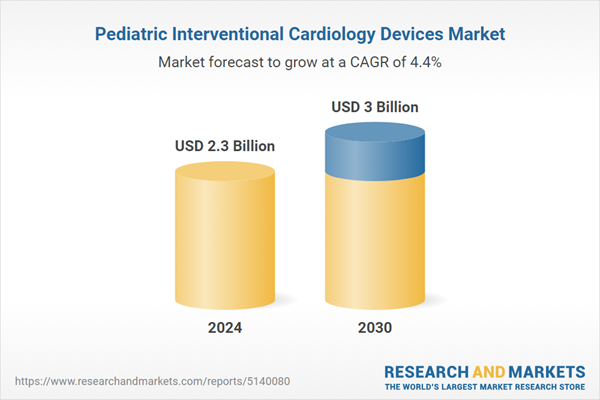
| Estimated Market Value ( USD | $ 2.3 Billion |
| Forecasted Market Value ( USD | $ 3 Billion |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |