Global Drug Device Combination Products Market - Key Trends & Drivers Summarized
What Are Drug Device Combination Products And Why Are They Important?
Drug device combination products are innovative therapeutic solutions that combine drugs, devices, and sometimes biologics to improve the efficacy, safety, and convenience of treatment for various medical conditions. These products are increasingly gaining importance in the healthcare industry as they offer enhanced treatment outcomes through synergistic effects, providing targeted drug delivery, controlled release, and reduced side effects. Common examples include drug-eluting stents, prefilled syringes, inhalers, transdermal patches, and implantable devices with drug coatings. The integration of drugs and devices is particularly valuable in chronic disease management, oncology, and cardiovascular treatments, where precision, control, and patient adherence are critical. As the demand for personalized and effective treatment options grows, drug device combination products are becoming an essential component of modern healthcare.How Are Technological Advancements Driving The Drug Device Combination Products Market?
Technological advancements are significantly driving the growth and development of the drug device combination products market. Innovations in drug delivery systems, such as microneedle patches, smart inhalers, and bioresorbable stents, are enhancing patient compliance and therapeutic effectiveness. The incorporation of digital health technologies, including sensors and wireless connectivity, is enabling real-time monitoring of patient adherence and drug delivery, providing valuable data to healthcare providers for optimizing treatment plans. Additionally, the development of novel materials, such as biocompatible polymers and hydrogels, is improving the safety and efficacy of implantable devices, reducing the risk of adverse reactions. The use of 3D printing technology in manufacturing combination products is also allowing for greater customization and precision, catering to the specific needs of individual patients. These technological advancements are transforming the market landscape by offering innovative solutions that address unmet clinical needs.How Are Regulatory Pathways And Market Dynamics Impacting Drug Device Combination Products?
The regulatory landscape for drug device combination products is complex, as these products must meet the standards of both drug and device regulations. Regulatory bodies such as the FDA and EMA are increasingly developing specific guidelines to streamline the approval process for combination products, ensuring safety and efficacy while reducing time to market. These regulatory frameworks are encouraging innovation by providing clearer pathways for the development and commercialization of combination products. Market dynamics, such as the rising prevalence of chronic diseases, the aging population, and the demand for minimally invasive treatments, are further driving the adoption of drug device combination products. The increasing focus on patient-centric care and the need for cost-effective healthcare solutions are also promoting the use of these products, as they offer improved treatment outcomes and reduced hospital stays.What Factors Are Driving The Growth Of The Drug Device Combination Products Market?
The growth in the drug device combination products market is driven by several factors, including technological advancements, favorable regulatory environments, and the rising prevalence of chronic diseases. A key driver is the increasing demand for minimally invasive treatments that offer better patient compliance and outcomes. The growing adoption of digital health technologies and smart drug delivery systems is also enhancing the effectiveness and convenience of combination products, making them more attractive to both healthcare providers and patients. Additionally, the expansion of healthcare infrastructure in emerging markets and the increasing focus on personalized medicine are supporting the development and adoption of these products. Strategic collaborations between pharmaceutical companies, medical device manufacturers, and research institutions are further propelling innovation and market growth. These factors, coupled with the continuous advancement of materials science and drug formulation technologies, are driving the global market for drug device combination products forward.Report Scope
The report analyzes the Drug Device Combination Products market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Transdermal Patches, Inhalers, Drug Eluting Stents, Prefilled Syringes, Photodynamic Therapy, Nebulizers, Antimicrobial Wound Dressings, Other Products); End-Use (Hospitals & Clinics, Home Care Setting, Ambulatory Surgery Centers, Other End-Uses).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Transdermal Patches segment, which is expected to reach US$53.4 Billion by 2030 with a CAGR of a 4.5%. The Inhalers segment is also set to grow at 5.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $31.5 Billion in 2024, and China, forecasted to grow at an impressive 8.3% CAGR to reach $36.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Drug Device Combination Products Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Drug Device Combination Products Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Drug Device Combination Products Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Alcon, a Novartis Company, Allergan PLC, Arrow International, Inc., Bausch & Lomb, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 46 companies featured in this Drug Device Combination Products market report include:
- Abbott Laboratories
- Alcon, a Novartis Company
- Allergan PLC
- Arrow International, Inc.
- Bausch & Lomb, Inc.
- Boston Scientific Corporation
- C.R. Bard, Inc.
- CareFusion Corporation
- Ethicon US LLC
- Medline Industries, Inc.
- Medtronic PLC
- Mylan NV
- Stryker Corporation
- Terumo Corporation
- W. L. Gore & Associates, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Alcon, a Novartis Company
- Allergan PLC
- Arrow International, Inc.
- Bausch & Lomb, Inc.
- Boston Scientific Corporation
- C.R. Bard, Inc.
- CareFusion Corporation
- Ethicon US LLC
- Medline Industries, Inc.
- Medtronic PLC
- Mylan NV
- Stryker Corporation
- Terumo Corporation
- W. L. Gore & Associates, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | January 2026 |
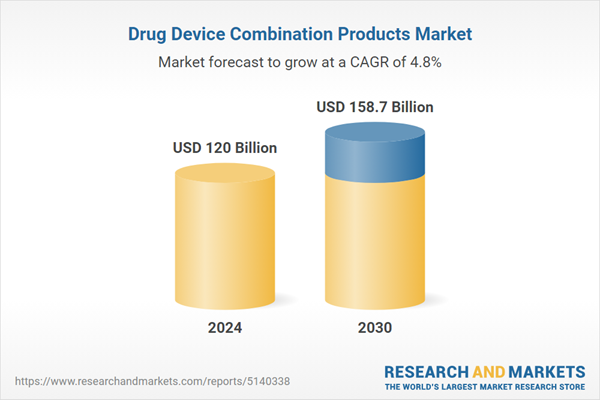
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 120 Billion |
| Forecasted Market Value ( USD | $ 158.7 Billion |
| Compound Annual Growth Rate | 4.8% |
| Regions Covered | Global |