Global Photopheresis Products Market - Key Trends & Drivers Summarized
Why Are Photopheresis Products Gaining Prominence as a Pioneering Therapy for Autoimmune Diseases and Graft-versus-Host Disease (GVHD)?
Photopheresis products are gaining prominence as a pioneering therapy for autoimmune diseases and Graft-versus-Host Disease (GVHD) due to their unique immunomodulatory effects and proven efficacy in treating conditions that are resistant to conventional therapies. Photopheresis, also known as extracorporeal photopheresis (ECP), is a specialized medical procedure that involves the collection of a patient's blood, separation of the white blood cells, exposure of these cells to a photosensitizing agent (such as 8-methoxypsoralen) and ultraviolet A (UVA) light, and re-infusion of the treated cells back into the patient's body. This process helps modify the immune response, promoting the generation of regulatory T cells and reducing inflammation, thereby offering therapeutic benefits in a range of autoimmune disorders and conditions associated with aberrant immune activity.The growing incidence of autoimmune diseases, including systemic sclerosis, cutaneous T-cell lymphoma (CTCL), and Crohn's disease, is driving the demand for photopheresis products as these therapies provide a targeted approach to managing these chronic and often debilitating conditions. In addition, photopheresis has emerged as an effective treatment option for both acute and chronic GVHD - a potentially life-threatening complication following allogeneic hematopoietic stem cell transplantation. For patients who do not respond to standard immunosuppressive therapies, photopheresis offers a safe and non-invasive alternative that helps reduce dependency on steroids and other immunosuppressive drugs. As awareness of the therapeutic potential of photopheresis continues to grow among healthcare providers and patients, the adoption of photopheresis products is expected to rise significantly.
How Are Technological Advancements Transforming the Photopheresis Products Market?
Technological advancements are transforming the photopheresis products market by enabling the development of more efficient, automated, and patient-friendly devices that improve therapeutic outcomes and streamline treatment processes. One of the most significant innovations in this space is the introduction of closed-system photopheresis devices, which minimize the risk of contamination and infection compared to traditional open systems. These closed systems integrate multiple steps of the photopheresis procedure, such as cell separation, irradiation, and re-infusion, into a single platform, enhancing safety and convenience for both patients and healthcare providers. The use of closed-system photopheresis devices is also reducing the need for extensive handling of blood components, decreasing treatment time, and improving patient comfort.Another transformative trend is the development of automated photopheresis systems that offer precise control over treatment parameters, such as the volume of blood processed, the duration of UVA exposure, and the concentration of the photosensitizing agent. These automated systems enable personalized treatment protocols tailored to the specific needs of individual patients, ensuring optimal therapeutic efficacy and minimizing side effects. The integration of digital interfaces, data recording capabilities, and remote monitoring features is further enhancing the usability and efficiency of photopheresis devices, allowing healthcare providers to track treatment progress, document patient outcomes, and make data-driven adjustments to therapy as needed.
The adoption of advanced light sources and photonic technologies is also influencing the growth of the photopheresis products market. Innovations such as light-emitting diode (LED)-based UVA light sources are offering greater control over light intensity and wavelength, ensuring consistent and uniform irradiation of blood components. These advancements are improving the safety and effectiveness of photopheresis treatments, particularly for patients with skin conditions such as CTCL. The use of sophisticated light delivery systems, including fiber-optic cables and specialized irradiation chambers, is enhancing the precision of photopheresis procedures, reducing variability in treatment outcomes, and supporting the development of next-generation photopheresis products.
Furthermore, the increasing use of software solutions and artificial intelligence (AI) in photopheresis devices is enabling real-time analysis of patient data, automated decision-making, and treatment optimization. AI algorithms can analyze large volumes of patient data to identify patterns and predict treatment responses, supporting personalized treatment plans and improving clinical outcomes. The integration of AI and machine learning (ML) in photopheresis products is helping healthcare providers make informed decisions, reduce the risk of human error, and enhance the overall safety and effectiveness of photopheresis therapy. As these technological advancements continue to evolve, they are making photopheresis products more sophisticated, efficient, and aligned with the needs of modern therapeutic practices.
What Role Do Market Dynamics and Regulatory Approvals Play in Shaping the Adoption of Photopheresis Products?
Market dynamics and regulatory approvals play a pivotal role in shaping the adoption of photopheresis products as healthcare providers and patients seek access to innovative therapies that meet stringent safety and efficacy standards. The rising incidence of autoimmune diseases, hematological malignancies, and transplant complications is driving demand for effective treatment options, creating a favorable market environment for photopheresis products. The growing awareness of the clinical benefits of photopheresis, such as its ability to modulate the immune system and promote long-term remission in patients with complex conditions, is encouraging healthcare providers to incorporate this therapy into their treatment protocols.Regulatory approvals are crucial for the commercialization and adoption of photopheresis products, as they provide assurance of product safety, quality, and efficacy. Regulatory agencies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and national health authorities have established specific guidelines for the approval of photopheresis devices and therapies. Achieving regulatory approval involves rigorous testing and clinical trials to demonstrate the safety and therapeutic benefits of photopheresis products. The FDA's approval of photopheresis for the treatment of CTCL, GVHD, and organ transplant rejection has set a precedent for the use of this therapy in immune-mediated conditions, supporting its adoption in clinical practice.
The expansion of reimbursement coverage for photopheresis treatments is also playing a significant role in driving market growth. In several countries, photopheresis is reimbursed for specific indications, such as GVHD and CTCL, making it more accessible to patients. The inclusion of photopheresis in insurance coverage plans and national health reimbursement schemes is reducing the financial burden on patients and encouraging healthcare providers to offer this therapy as part of standard care. The availability of reimbursement is particularly important for promoting the adoption of photopheresis products in regions with high healthcare costs, as it ensures that patients can access potentially life-saving treatments without incurring prohibitive expenses.
The role of industry collaborations and partnerships is also influencing the development and commercialization of photopheresis products. Collaborations between medical device manufacturers, research institutions, and healthcare providers are facilitating the advancement of photopheresis technologies, the conduct of clinical trials, and the generation of real-world evidence to support product adoption. These partnerships are enabling the development of innovative photopheresis products with improved performance and usability, as well as the exploration of new therapeutic applications. The establishment of clinical networks and treatment centers specializing in photopheresis is supporting the dissemination of knowledge, best practices, and clinical expertise, further promoting the adoption of photopheresis products in the medical community.
What Factors Are Driving the Growth of the Global Photopheresis Products Market?
The growth in the global photopheresis products market is driven by several factors, including the increasing prevalence of autoimmune diseases, the rising demand for effective therapies to manage GVHD, and ongoing advancements in photopheresis technology. One of the primary growth drivers is the growing incidence of autoimmune disorders, such as systemic lupus erythematosus (SLE), multiple sclerosis, and scleroderma, which are characterized by the immune system attacking the body's own tissues. These chronic conditions often require long-term management and are associated with significant morbidity and reduced quality of life. Photopheresis offers a novel therapeutic approach that modulates the immune system without the need for high doses of immunosuppressive drugs, making it a valuable option for patients who do not respond adequately to conventional therapies.The increasing demand for effective therapies to manage GVHD is another key factor contributing to market growth. GVHD is a major complication of allogeneic stem cell transplantation, where donor immune cells attack the recipient's tissues. This condition can affect multiple organs and is associated with high morbidity and mortality rates. Photopheresis has been shown to be effective in both preventing and treating acute and chronic GVHD by inducing immune tolerance and promoting the generation of regulatory T cells. The ability of photopheresis to provide therapeutic benefits in GVHD patients without causing significant immunosuppression is driving its adoption as a standard of care in many transplant centers.
Ongoing advancements in photopheresis technology are further supporting the growth of the market. The development of next-generation photopheresis devices with improved automation, safety features, and treatment efficiency is enhancing the overall patient experience and clinical outcomes. The introduction of portable and compact photopheresis systems is expanding the availability of this therapy to smaller healthcare facilities and outpatient settings, increasing patient access. Innovations such as closed-system devices, automated cell processing, and advanced irradiation technologies are making photopheresis more accessible and convenient for both patients and healthcare providers.
Moreover, the growing focus on personalized medicine and targeted therapies is creating new opportunities for photopheresis products. Photopheresis offers the potential for personalized treatment protocols that can be tailored to the specific needs of individual patients based on their disease characteristics and treatment response. The use of biomarkers and diagnostic tools to identify patients who are most likely to benefit from photopheresis is supporting the development of personalized treatment strategies that maximize therapeutic efficacy and minimize adverse effects. As the field of personalized medicine continues to evolve, the demand for photopheresis products that enable individualized treatment approaches is expected to grow.
Additionally, the increasing investment in research and development (R&D) and the expansion of clinical trials for new indications are contributing to market growth. Pharmaceutical and medical device companies are investing in the development of photopheresis products for new therapeutic areas, such as solid organ transplantation, autoimmune diseases, and dermatological conditions. The positive outcomes of clinical trials and the publication of real-world evidence are supporting the use of photopheresis in a broader range of applications, creating new opportunities for market expansion.
Furthermore, the impact of the COVID-19 pandemic has highlighted the importance of immunomodulatory therapies in managing severe immune responses. Photopheresis has been explored as a potential treatment option for managing cytokine storm and hyperinflammation in COVID-19 patients, providing a new perspective on its therapeutic potential. The increased interest in immunomodulatory therapies during the pandemic has raised awareness of photopheresis and its potential applications beyond traditional indications. As these factors converge, the global photopheresis products market is poised for robust growth, driven by technological advancements, expanding clinical applications, and the increasing emphasis on developing innovative therapies to address complex immune-mediated conditions.
Report Scope
The report analyzes the Photopheresis Products market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Application (Cutaneous T-cell Lymphoma, Graft versus Host Disease, Transplant Rejections, Autoimmune Diseases); Product Type (Open System, Closed System).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Open System segment, which is expected to reach US$164.4 Million by 2030 with a CAGR of a 5.1%. The Closed System segment is also set to grow at 5.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $121.1 Million in 2024, and China, forecasted to grow at an impressive 5.4% CAGR to reach $96.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Photopheresis Products Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Photopheresis Products Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Photopheresis Products Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AdventHealth Medical Group, ANTISEL, Fresenius Kabi AG, Haemonetics Corporation, MacoPharma and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 58 companies featured in this Photopheresis Products market report include:
- AdventHealth Medical Group
- ANTISEL
- Fresenius Kabi AG
- Haemonetics Corporation
- MacoPharma
- Mallinckrodt Pharmaceuticals
- Med Tech Solutions GmbH
- PIT Medical Systems GmbH
- Terumo Corporation
- Therakos
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AdventHealth Medical Group
- ANTISEL
- Fresenius Kabi AG
- Haemonetics Corporation
- MacoPharma
- Mallinckrodt Pharmaceuticals
- Med Tech Solutions GmbH
- PIT Medical Systems GmbH
- Terumo Corporation
- Therakos
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 231 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
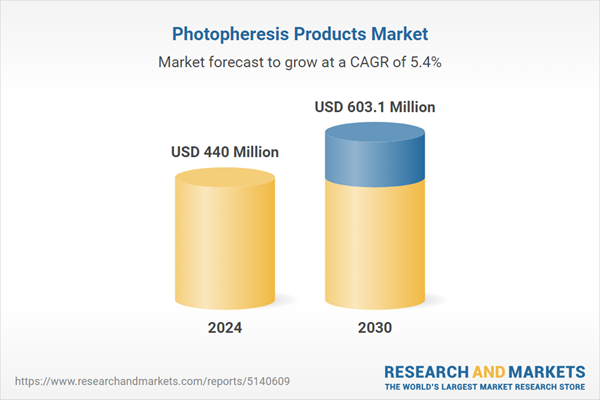
| Estimated Market Value ( USD | $ 440 Million |
| Forecasted Market Value ( USD | $ 603.1 Million |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |