Global Hydrophobic Interaction Chromatography Market - Key Trends and Drivers Summarized
Is Hydrophobic Interaction Chromatography the Game-Changer for Protein Purification and Biopharmaceuticals?
Hydrophobic interaction chromatography (HIC) is a vital technique in protein purification, but why is it so critical for biopharmaceutical development, biochemical research, and industrial protein production? Hydrophobic interaction chromatography is a powerful method used to separate and purify proteins, peptides, and other biomolecules based on their hydrophobicity. In HIC, biomolecules are applied to a chromatography column that contains a hydrophobic stationary phase. By manipulating the salt concentration in the mobile phase, researchers can control how proteins bind to or elute from the hydrophobic surface, allowing for precise separation of complex mixtures.The significance of HIC lies in its ability to purify proteins without denaturing them, which is crucial for maintaining their biological activity. This makes HIC a preferred technique in the biopharmaceutical industry, where proteins, antibodies, and other biomolecules need to be purified for therapeutic use. HIC is particularly effective in downstream processing, where it is used to remove impurities and contaminants from recombinant proteins and monoclonal antibodies. By providing a reliable and efficient method for purifying complex biomolecules, hydrophobic interaction chromatography is central to the production of high-purity proteins essential for drug development, diagnostics, and research.
How Have Technological Advancements Improved Hydrophobic Interaction Chromatography for Biotech and Pharma?
Technological advancements have significantly enhanced hydrophobic interaction chromatography, making it more effective, scalable, and adaptable for modern biopharmaceutical and research applications. One of the key advancements has been the development of high-performance resins and stationary phases with optimized hydrophobic ligands. These resins improve binding specificity and capacity, allowing for more efficient separation of proteins with varying degrees of hydrophobicity. Modern HIC resins offer greater resolution, enabling the purification of closely related biomolecules, such as protein isoforms and antibody variants, which is critical in the production of biotherapeutics where purity is paramount.Another important development in HIC technology is the ability to tailor the hydrophobicity of the stationary phase to suit specific biomolecules. Advances in resin chemistry have made it possible to adjust the degree of hydrophobicity by modifying ligand types and densities, allowing researchers to fine-tune the separation process. This flexibility is especially valuable in applications like monoclonal antibody purification, where slight variations in hydrophobicity can impact the final product's efficacy. Tailored HIC resins ensure that proteins are purified under mild conditions, preserving their native conformation and biological activity, which is crucial for therapeutic proteins.
Automated HIC systems have also emerged as a major technological advancement, improving the efficiency and reproducibility of the chromatography process. Automated systems allow for precise control of flow rates, salt gradients, and column loading, ensuring that the separation process is highly controlled and reproducible. Automation reduces human error and variability, which is essential in large-scale biopharmaceutical production where consistency in product purity is critical. The integration of HIC into automated systems has streamlined downstream processing in biomanufacturing, allowing for faster and more scalable protein purification workflows.
Advancements in mixed-mode chromatography, which combines hydrophobic interaction with other separation techniques like ion exchange or affinity chromatography, have further expanded the applications of HIC. These hybrid approaches allow for the simultaneous separation of biomolecules based on multiple characteristics, such as charge and hydrophobicity. Mixed-mode HIC has proven especially useful in purifying complex biologics like fusion proteins, antibody-drug conjugates (ADCs), and other multimodal therapeutic agents. This advancement provides a more robust and flexible purification process, reducing the number of purification steps and enhancing overall efficiency in bioprocessing.
High-throughput screening methods have also been developed to accelerate the optimization of HIC conditions. These methods allow researchers to quickly evaluate multiple resin types, buffer conditions, and salt gradients in parallel, identifying the optimal settings for purifying a particular biomolecule. High-throughput techniques are particularly valuable in early-stage drug development, where time and resource efficiency are critical. By speeding up the process of condition optimization, these techniques help bring biopharmaceutical products to market faster while ensuring high levels of purity and quality.
Another significant advancement is the development of scalable HIC platforms that can be easily adapted from laboratory-scale purification to large-scale industrial production. This scalability is particularly important in biopharmaceutical manufacturing, where the need to produce large quantities of purified proteins or antibodies requires chromatography systems that maintain performance as they scale up. Scalable HIC resins and systems allow for a seamless transition from research to commercial production, ensuring that the purification process remains consistent and reliable, regardless of the production volume.
Why Is Hydrophobic Interaction Chromatography Critical for Protein Purification and Biopharmaceutical Production?
Hydrophobic interaction chromatography is critical for protein purification and biopharmaceutical production because it offers a unique method of separating biomolecules based on hydrophobic interactions while preserving the native structure and function of proteins. One of the main reasons HIC is so important is its ability to purify proteins without subjecting them to harsh conditions that could denature or deactivate them. Many proteins, especially those used in biopharmaceuticals, are sensitive to changes in temperature, pH, or ionic strength. HIC operates under relatively mild conditions, using aqueous buffer systems that maintain protein stability throughout the purification process. This makes it an ideal technique for purifying therapeutic proteins that need to retain their biological activity.HIC is also crucial for the purification of monoclonal antibodies (mAbs), which are widely used in the treatment of various diseases, including cancer and autoimmune disorders. Monoclonal antibodies often require highly specific purification processes to ensure that they are free from contaminants such as host cell proteins, DNA, and process-related impurities. HIC is particularly effective in separating antibodies from aggregates, fragments, and other forms of misfolded proteins, ensuring the production of high-purity biotherapeutics. The precision offered by HIC makes it a vital part of the downstream processing of antibodies, contributing to the safety and efficacy of these drugs.
In addition to therapeutic proteins and antibodies, HIC is also used in the purification of vaccine components, enzymes, and other biopharmaceuticals. The ability of HIC to separate proteins based on subtle differences in hydrophobicity is particularly valuable in cases where high-purity separation is needed, such as in the production of recombinant proteins. Many recombinant proteins are expressed in host cells like bacteria or yeast, where they must be isolated from a complex mixture of host cell proteins and other impurities. HIC provides an efficient way to purify these proteins, removing unwanted components while maintaining the activity of the target molecule.
Another reason HIC is essential for biopharmaceutical production is its compatibility with other chromatographic techniques. In bioprocessing, multiple chromatography steps are often used in sequence to achieve the desired level of purity. HIC is commonly used after initial purification steps such as affinity chromatography or ion exchange chromatography to further refine the product. Its ability to complement other techniques makes HIC a versatile tool for building robust purification strategies that can be tailored to specific biomolecules. By integrating HIC with other purification methods, manufacturers can achieve higher yields, greater purity, and more efficient production processes.
In research and development, HIC plays a key role in protein characterization and structural studies. The technique can be used to analyze the hydrophobicity of proteins and their interaction with hydrophobic surfaces, providing valuable insights into protein folding, stability, and function. These insights are critical for drug discovery and development, where understanding the behavior of therapeutic proteins under different conditions can guide the design of more effective treatments. HIC's ability to separate proteins based on hydrophobicity also makes it a valuable tool for studying protein-protein interactions, aggregation tendencies, and the effects of post-translational modifications.
In industrial applications, HIC is used for the large-scale purification of proteins, enzymes, and other biologics that require stringent quality control. The scalability of HIC systems, along with their ability to consistently produce high-purity products, makes them indispensable in the commercial production of biopharmaceuticals. As the demand for biologic drugs continues to grow, HIC provides an efficient and reliable method for meeting production needs while maintaining the safety and efficacy of the final product. Its role in ensuring product quality and consistency is critical for regulatory compliance and market approval of biopharmaceuticals.
What Factors Are Driving the Growth of the Hydrophobic Interaction Chromatography Market?
Several factors are driving the rapid growth of the hydrophobic interaction chromatography market, including the increasing demand for biopharmaceuticals, advancements in protein-based drug development, and the growing focus on biologics in medical treatments. One of the primary drivers is the expanding biopharmaceutical industry, where the need for high-purity proteins, antibodies, and vaccines is growing. Biologics have become a key area of focus in drug development, particularly in therapies for cancer, autoimmune diseases, and infectious diseases. As the production of these biologics requires precise and efficient purification processes, the demand for HIC systems is rising.The growing adoption of monoclonal antibodies in the treatment of various medical conditions is another significant factor driving the HIC market. Monoclonal antibodies are among the fastest-growing classes of therapeutic drugs, and their production requires rigorous purification to ensure safety and efficacy. HIC plays a critical role in purifying monoclonal antibodies by separating them from impurities, aggregates, and unwanted forms, making it an indispensable tool in their manufacturing. As the market for antibody-based therapies continues to expand, the demand for HIC is expected to rise accordingly.
The increasing complexity of biopharmaceutical products, such as antibody-drug conjugates (ADCs), fusion proteins, and biosimilars, is also contributing to the growth of the hydrophobic interaction chromatography market. These complex biologics require sophisticated purification strategies to ensure the separation of different molecular forms and achieve the desired purity levels. HIC's ability to resolve proteins and biomolecules based on their hydrophobicity makes it an ideal technique for purifying these advanced therapeutics. As biopharmaceutical companies continue to innovate and develop new classes of biologic drugs, HIC will play an increasingly important role in their production.
Technological advancements in chromatography resins and systems are further fueling the growth of the HIC market. The development of more efficient and high-capacity resins has improved the resolution and speed of protein purification, making HIC more effective and scalable for industrial use. In addition, the integration of automated systems has made HIC more accessible to companies by reducing manual intervention and improving reproducibility. These technological innovations are making HIC a more attractive option for both large-scale biopharmaceutical manufacturers and smaller biotech firms engaged in drug development.
The rise of biosimilars - biopharmaceutical products that are highly similar to already-approved biologics - is also driving demand for efficient purification methods like HIC. As biosimilar manufacturers seek to develop cost-effective production processes while maintaining the quality and safety of their products, HIC offers a reliable solution for ensuring high-purity protein production. With the growing approval and adoption of biosimilars in global markets, the demand for HIC systems is expected to increase.
Lastly, regulatory requirements for biopharmaceuticals are placing greater emphasis on product purity and quality, driving the need for advanced purification technologies. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require biopharmaceutical manufacturers to demonstrate that their products meet stringent purity standards. HIC is widely recognized as a robust and reliable technique for achieving these standards, making it a critical component of the biomanufacturing process.
With the rising demand for biologic therapies, the growth of the biopharmaceutical industry, and advancements in protein purification technologies, the hydrophobic interaction chromatography market is poised for continued expansion. As biopharmaceutical companies seek to optimize their purification workflows and produce high-purity products efficiently, HIC will remain an essential tool in the production of next-generation biologics.
Report Scope
The report analyzes the Hydrophobic Interaction Chromatography market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Product & Services (Resins, Columns, Buffers, Other Product & Services); Sample (Monoclonal Antibodies, Vaccines, Other Samples).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Resins segment, which is expected to reach US$263.1 Million by 2030 with a CAGR of 7.3%. The Buffers segment is also set to grow at 6.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $128.9 Million in 2024, and China, forecasted to grow at an impressive 9.6% CAGR to reach $170.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Hydrophobic Interaction Chromatography Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Hydrophobic Interaction Chromatography Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Hydrophobic Interaction Chromatography Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Avantor Performance Materials LLC, Bio-Rad Laboratories, Inc., Danaher Corporation, GE Healthcare Life Sciences, Geno Technology Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 11 companies featured in this Hydrophobic Interaction Chromatography market report include:
- Avantor Performance Materials LLC
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- GE Healthcare Life Sciences
- Geno Technology Inc.
- JNC Corporation
- Knauer GmbH
- Merck KgaA
- Mitsubishi Chemical Corporation
- Sartorius AG
- Sepax Technologies, Inc.
- Thermo Fisher Scientific, Inc.
- Tosoh Corporation
- Waters Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Avantor Performance Materials LLC
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- GE Healthcare Life Sciences
- Geno Technology Inc.
- JNC Corporation
- Knauer GmbH
- Merck KgaA
- Mitsubishi Chemical Corporation
- Sartorius AG
- Sepax Technologies, Inc.
- Thermo Fisher Scientific, Inc.
- Tosoh Corporation
- Waters Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 179 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
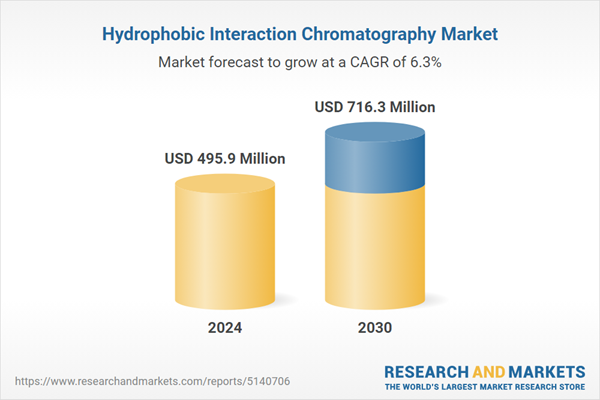
| Estimated Market Value ( USD | $ 495.9 Million |
| Forecasted Market Value ( USD | $ 716.3 Million |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |