Global Immunoprotein Diagnostic Testing Market - Key Trends & Drivers Summarized
Why is Immunoprotein Diagnostic Testing Emerging as a Vital Tool in Disease Diagnosis and Management?
Immunoprotein diagnostic testing has become an indispensable tool in the early detection, diagnosis, and monitoring of various diseases and health conditions, including autoimmune disorders, infectious diseases, cardiovascular diseases, and cancer. These tests are designed to measure the levels of specific immunoproteins - such as immunoglobulins (IgA, IgG, IgM), C-reactive protein (CRP), complement proteins, and rheumatoid factors - in blood, serum, or plasma samples. By detecting abnormal concentrations of these proteins, immunoprotein diagnostic tests provide crucial insights into the presence and progression of inflammatory responses, immune system activity, and disease states. The increasing prevalence of chronic and autoimmune diseases, along with the growing need for precise and reliable diagnostic tools, is driving the adoption of immunoprotein testing across clinical laboratories, hospitals, and research institutions worldwide.The global immunoprotein diagnostic testing market is further fueled by advancements in laboratory automation, the rising demand for point-of-care testing (POCT), and the growing awareness of preventive healthcare. Technological innovations such as automated immunoassay analyzers and multiplex testing platforms are enabling faster, more accurate, and cost-effective diagnostic processes, enhancing the efficiency and productivity of clinical laboratories. Moreover, the increasing focus on preventive healthcare and early disease detection is boosting the demand for immunoprotein diagnostic tests as they provide valuable information that helps healthcare providers initiate timely interventions. The expanding applications of immunoprotein diagnostics in oncology, infectious disease management, and personalized medicine are positioning this market as a critical component of modern healthcare, supporting better patient outcomes and more informed clinical decision-making.
What Technological Advancements Are Transforming the Immunoprotein Diagnostic Testing Market?
Technological advancements are significantly transforming the immunoprotein diagnostic testing market by enhancing the accuracy, speed, and convenience of testing procedures. One of the most impactful innovations is the development of high-sensitivity and high-specificity immunoassays, such as enzyme-linked immunosorbent assays (ELISA), chemiluminescence immunoassays (CLIA), and radioimmunoassays (RIA). These advanced assay technologies enable the detection of even minute concentrations of immunoproteins, making it possible to diagnose diseases at very early stages when clinical symptoms may not yet be apparent. The introduction of automated immunoassay analyzers that can perform multiple tests simultaneously is streamlining workflow in clinical laboratories, reducing turnaround times, and minimizing the potential for human error. These systems are also capable of handling high-throughput testing, making them ideal for large-scale screening programs and hospital settings.Another transformative technological advancement is the rise of point-of-care testing (POCT) devices that allow for rapid immunoprotein diagnostics at the patient's bedside, in outpatient settings, or even at home. POCT devices are designed to be user-friendly, portable, and capable of delivering accurate results within minutes, facilitating faster clinical decisions and reducing the need for centralized laboratory testing. The use of lateral flow assays, biosensors, and microfluidic devices in POCT is expanding the accessibility of immunoprotein diagnostics to a wider population, particularly in rural and underserved areas where access to traditional laboratory facilities may be limited. Additionally, the integration of digital health technologies, such as connectivity features and data-sharing capabilities, is enabling real-time monitoring and analysis of immunoprotein levels, supporting telemedicine and remote patient management.
Further innovations include the application of multiplex testing platforms and biochip technologies, which allow for the simultaneous measurement of multiple immunoproteins from a single sample. These technologies are providing a more comprehensive understanding of disease states by offering a detailed profile of the patient's immune response. The integration of artificial intelligence (AI) and machine learning (ML) algorithms is also enhancing the interpretation of complex immunoprotein data, identifying patterns, and predicting disease risk with greater accuracy. AI-driven diagnostic platforms can analyze large datasets and provide clinicians with actionable insights, supporting the shift towards personalized and precision medicine. These technological advancements are revolutionizing the field of immunoprotein diagnostic testing, making it possible to deliver faster, more reliable, and more comprehensive diagnostic solutions that cater to the evolving needs of modern healthcare.
How Are Market Dynamics and Regulatory Standards Influencing the Immunoprotein Diagnostic Testing Market?
The immunoprotein diagnostic testing market is shaped by a complex set of market dynamics and regulatory standards that are influencing product development, adoption, and accessibility. One of the primary market drivers is the increasing prevalence of chronic diseases, autoimmune disorders, and infections, which is creating a growing demand for diagnostic tools that can provide early and accurate disease detection. As the global burden of diseases such as rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis continues to rise, there is a heightened need for immunoprotein diagnostic tests that can assess immune function, monitor disease activity, and evaluate treatment efficacy. In oncology, immunoprotein markers are being used to detect and monitor certain types of cancers, such as prostate cancer (using prostate-specific antigen, or PSA) and multiple myeloma (using beta-2 microglobulin), providing valuable information for guiding treatment decisions and assessing prognosis.Regulatory standards and compliance requirements are also playing a critical role in shaping the immunoprotein diagnostic testing market. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established stringent guidelines for the development, approval, and commercialization of diagnostic tests to ensure their safety, efficacy, and reliability. Compliance with these regulations is essential for manufacturers to gain market access and maintain the trust of healthcare providers and patients. The regulatory landscape is evolving to keep pace with advances in diagnostic technologies, particularly with regard to point-of-care testing and digital health solutions. For instance, the FDA's Emergency Use Authorization (EUA) process, which was widely used during the COVID-19 pandemic to expedite the availability of diagnostic tests, has highlighted the need for flexible and responsive regulatory frameworks that can support innovation while ensuring patient safety. As new diagnostic technologies and platforms emerge, regulatory agencies are refining their policies to address issues such as data privacy, cybersecurity, and clinical validation, which are critical for ensuring the quality and integrity of immunoprotein diagnostic tests.
Market dynamics such as competition among manufacturers, pricing pressures, and healthcare reimbursement policies are also influencing the immunoprotein diagnostic testing market. The competitive landscape is characterized by the presence of both established players and new entrants, each striving to develop novel diagnostic solutions and gain a foothold in this rapidly evolving market. Companies are differentiating themselves through product innovation, strategic partnerships, and the ability to offer comprehensive diagnostic solutions that include not only testing devices but also software, data analytics, and support services. Pricing pressures, particularly in cost-sensitive markets, are driving the demand for cost-effective diagnostic tests that offer high performance without compromising quality. Additionally, healthcare reimbursement policies and coverage decisions by payers are impacting the adoption of immunoprotein diagnostic tests. The reimbursement environment varies significantly across regions and is influenced by factors such as the clinical utility of the test, the availability of alternative diagnostic options, and the overall healthcare budget. Navigating these market dynamics and regulatory standards is essential for companies operating in the immunoprotein diagnostic testing market as they seek to expand their presence and address the unmet diagnostic needs of patients and healthcare providers.
What Are the Key Growth Drivers Fueling the Expansion of the Immunoprotein Diagnostic Testing Market?
The growth in the global immunoprotein diagnostic testing market is driven by several key factors, including the rising incidence of chronic and autoimmune diseases, increasing focus on early disease detection and preventive healthcare, and advancements in diagnostic technologies. One of the primary growth drivers is the growing prevalence of chronic and autoimmune diseases, which is creating a significant demand for diagnostic tests that can provide early and accurate detection. Autoimmune diseases, in particular, are often difficult to diagnose due to their complex and overlapping symptoms. Immunoprotein diagnostic tests offer a reliable means of detecting autoimmune markers and assessing disease activity, enabling earlier diagnosis and more effective disease management. As the global population ages and the incidence of these conditions continues to rise, the demand for immunoprotein diagnostics is expected to increase, supporting market expansion.Another significant growth driver is the increasing focus on early disease detection and preventive healthcare. Early detection of diseases such as cardiovascular disease, diabetes, and cancer can significantly improve treatment outcomes and reduce healthcare costs. Immunoprotein diagnostic tests, which can detect early biomarkers of inflammation, infection, and malignancy, are being used to screen for risk factors and monitor disease progression. The growing emphasis on preventive healthcare is encouraging healthcare providers and patients to adopt diagnostic tests that can identify health issues before they become clinically apparent. This trend is particularly strong in developed regions such as North America and Europe, where healthcare systems are increasingly prioritizing preventive care and wellness initiatives. Additionally, the rise of personalized medicine is driving the use of immunoprotein diagnostics to tailor treatment strategies based on individual patient profiles, further supporting market growth.
Technological advancements in diagnostic testing are also fueling the growth of the immunoprotein diagnostic testing market. Innovations such as high-throughput immunoassay platforms, point-of-care testing devices, and digital health solutions are making diagnostic tests more accessible, accurate, and user-friendly. The development of multiplex testing platforms that can measure multiple biomarkers from a single sample is providing a more comprehensive view of disease states, enabling more precise diagnosis and monitoring. The adoption of digital technologies, such as connectivity features and data-sharing capabilities, is enhancing the integration of immunoprotein diagnostics into broader healthcare ecosystems, supporting telemedicine and remote patient management. As technological innovations continue to improve the performance and accessibility of immunoprotein diagnostic tests, and as the focus on early detection and personalized medicine grows, the global immunoprotein diagnostic testing market is poised for sustained growth, driven by increasing demand from healthcare providers and patients seeking more effective diagnostic solutions.
Report Scope
The report analyzes the Immunoprotein Diagnostic Testing market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Immunoglobulin Diagnostic, Free Light Chain Diagnostic, Pre-Albumin Diagnostic, Haptoglobin Diagnostic, C-Reactive Protein Diagnostic, Complement System Protein Diagnostic, Other Types); Technology (Enzyme-Linked Immunosorbent Assay, Immunoturbidity Assay, Radioimmunoassay, Immunoprotein Electrophoresis, Other Technologies); Application (Infectious Disease Testing, Oncology Testing, Autoimmune Testing, Endocrine Testing, Allergy Testing, Toxicology Testing, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Immunoglobulin Diagnostic Testing segment, which is expected to reach US$6.4 Billion by 2030 with a CAGR of 6%. The Free Light Chain Diagnostic Testing segment is also set to grow at 4.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.6 Billion in 2024, and China, forecasted to grow at an impressive 4.8% CAGR to reach $3.5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Immunoprotein Diagnostic Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Immunoprotein Diagnostic Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Immunoprotein Diagnostic Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Abcam PLC, Bio-Rad Laboratories, Inc., Danaher Corporation, DiaSorin SpA and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 38 companies featured in this Immunoprotein Diagnostic Testing market report include:
- Abbott Laboratories
- Abcam PLC
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- DiaSorin SpA
- Enzo Biochem, Inc.
- F. Hoffmann-La Roche AG
- Ortho Clinical Diagnostics
- Siemens AG
- Thermo Fisher Scientific, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Abcam PLC
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- DiaSorin SpA
- Enzo Biochem, Inc.
- F. Hoffmann-La Roche AG
- Ortho Clinical Diagnostics
- Siemens AG
- Thermo Fisher Scientific, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 171 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
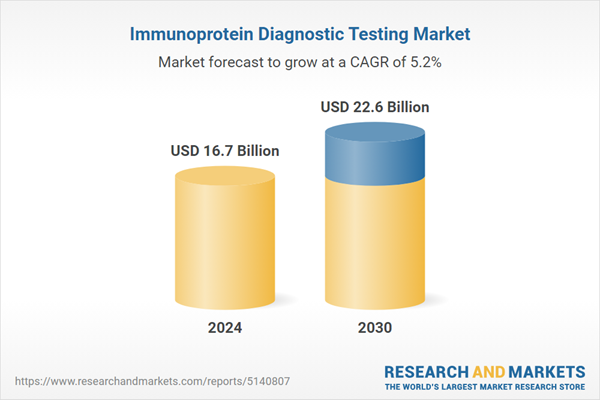
| Estimated Market Value ( USD | $ 16.7 Billion |
| Forecasted Market Value ( USD | $ 22.6 Billion |
| Compound Annual Growth Rate | 5.2% |
| Regions Covered | Global |