Global Enteric Disease Testing Market - Key Trends and Drivers Summarized
How Is Enteric Disease Testing Revolutionizing Public Health and Disease Management?
Enteric disease testing is revolutionizing public health and disease management by providing rapid, accurate, and comprehensive tools for diagnosing infections that affect the gastrointestinal tract. Enteric diseases, caused by bacteria, viruses, or parasites, are a significant global health concern, particularly in areas with limited access to clean water and sanitation. These diseases can lead to severe dehydration, malnutrition, and, in some cases, death, especially among vulnerable populations such as children and the elderly. The ability to quickly and accurately diagnose enteric infections is critical for effective treatment, containment, and prevention of outbreaks. Modern enteric disease testing leverages advanced diagnostic technologies, including molecular assays, immunoassays, and next-generation sequencing, to detect a wide range of pathogens with high sensitivity and specificity. By enabling healthcare providers to identify the exact cause of gastrointestinal symptoms promptly, enteric disease testing is improving patient outcomes, reducing the spread of infections, and supporting public health initiatives aimed at controlling and preventing enteric diseases worldwide.What Innovations Are Enhancing the Functionality of Enteric Disease Testing?
Innovations in enteric disease testing are enhancing its functionality through advancements in molecular diagnostics, point-of-care testing, and multiplex testing platforms. One of the most significant developments is the use of polymerase chain reaction (PCR) and other nucleic acid amplification techniques, which allow for the rapid detection of genetic material from pathogens with high accuracy. These molecular diagnostic methods can identify specific bacteria, viruses, and parasites in stool samples within hours, compared to traditional culture methods that may take days. The speed and precision of molecular diagnostics are particularly valuable in managing outbreaks and ensuring that patients receive the appropriate treatment quickly, thereby reducing the risk of complications and transmission.Point-of-care testing (POCT) is another key innovation that is transforming enteric disease diagnostics. POCT devices are designed for use at the bedside or in remote settings, providing immediate results without the need for specialized laboratory equipment. These portable, easy-to-use devices are particularly beneficial in resource-limited areas where access to traditional laboratory infrastructure may be lacking. POCT allows for the rapid diagnosis and treatment of enteric diseases in the field, reducing the time to care and improving the chances of a successful outcome. This innovation is also critical in outbreak situations, where timely identification of the causative pathogen can prevent the spread of disease.
Multiplex testing platforms represent another major advancement in enteric disease testing. These systems can simultaneously detect multiple pathogens from a single sample, providing a comprehensive analysis of potential causes of gastrointestinal symptoms. Multiplex tests are particularly useful in cases where symptoms could be caused by a variety of pathogens, such as in foodborne illness outbreaks or in immunocompromised patients. By providing a broad-spectrum diagnostic tool, multiplex testing reduces the need for multiple individual tests, saving time and resources while ensuring a thorough investigation of the patient's condition.
These innovations are making enteric disease testing more rapid, accurate, and accessible, which is crucial for effective disease management and the protection of public health.
How Does Enteric Disease Testing Impact Public Health and Patient Outcomes?
Enteric disease testing has a profound impact on public health and patient outcomes by enabling the timely diagnosis, treatment, and prevention of infections that affect the gastrointestinal system. For individual patients, accurate and rapid testing is critical for identifying the specific pathogen responsible for their illness, which guides appropriate treatment. Without proper diagnosis, patients may receive ineffective or inappropriate treatments, leading to prolonged illness, increased risk of complications, and higher healthcare costs. Enteric disease testing allows for targeted therapy, reducing the duration and severity of the illness and minimizing the potential for complications such as dehydration, especially in vulnerable populations like children and the elderly.From a public health perspective, enteric disease testing is essential for controlling the spread of infectious diseases. Rapid identification of pathogens in community outbreaks allows public health authorities to implement containment measures more effectively, such as isolating infected individuals, issuing public warnings, and tracing the source of contamination. In the case of foodborne illnesses, prompt testing can lead to the identification of contaminated food products, preventing further cases and protecting public health. Moreover, the data generated from enteric disease testing contribute to surveillance efforts, helping to track trends in disease incidence, monitor emerging pathogens, and evaluate the effectiveness of public health interventions.
Enteric disease testing also plays a vital role in global health, particularly in regions with high burdens of gastrointestinal infections. In areas where access to clean water and sanitation is limited, enteric diseases are a leading cause of morbidity and mortality. By providing the tools needed for early detection and treatment, enteric disease testing helps to reduce the burden of these diseases and supports broader efforts to improve health outcomes in vulnerable populations. Additionally, the availability of rapid and accurate testing is crucial in humanitarian settings, such as refugee camps, where outbreaks of enteric diseases can spread quickly and have devastating consequences.
Overall, the impact of enteric disease testing on public health is far-reaching, improving patient outcomes, enhancing disease control efforts, and contributing to the prevention of future outbreaks.
What Trends Are Driving Growth in the Enteric Disease Testing Market?
Several trends are driving growth in the enteric disease testing market, including the increasing incidence of foodborne illnesses, advancements in diagnostic technology, the rising demand for rapid testing solutions, and global public health initiatives aimed at controlling infectious diseases. The growing incidence of foodborne illnesses is a significant factor fueling the demand for enteric disease testing. As globalization of the food supply chain increases, the risk of widespread outbreaks caused by contaminated food products has risen, prompting greater emphasis on food safety and the need for reliable testing methods to quickly identify and contain such outbreaks.Advancements in diagnostic technology, particularly in molecular diagnostics, are also contributing to the growth of the enteric disease testing market. The development of faster, more accurate, and cost-effective diagnostic tools has expanded the availability and accessibility of enteric disease testing. Innovations such as PCR-based assays, next-generation sequencing, and multiplex platforms have made it possible to detect multiple pathogens simultaneously with high sensitivity and specificity, even in low-resource settings. These advancements are not only improving patient care but also supporting broader public health efforts by providing essential data for surveillance and outbreak response.
The rising demand for rapid testing solutions is another key trend driving market growth. In both clinical and public health settings, there is a growing need for diagnostic tests that can deliver results quickly, allowing for prompt treatment and intervention. This demand is particularly acute in emergency situations, such as outbreaks or natural disasters, where timely diagnosis is critical to preventing the spread of disease and saving lives. The development and deployment of point-of-care testing devices that provide rapid, accurate results in the field are meeting this need and driving adoption in diverse settings.
Global public health initiatives aimed at reducing the burden of enteric diseases, particularly in low- and middle-income countries, are also driving growth in the enteric disease testing market. Organizations such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) are investing in programs to improve access to diagnostics, strengthen disease surveillance systems, and enhance the capacity for rapid response to outbreaks. These efforts are increasing the demand for advanced enteric disease testing solutions that can support these goals.
Additionally, the focus on antimicrobial resistance (AMR) is influencing the enteric disease testing market. With the growing concern over the misuse of antibiotics and the rise of resistant pathogens, there is an increasing emphasis on accurate diagnosis to ensure appropriate treatment. Enteric disease testing that can rapidly identify the causative pathogen and its resistance profile is becoming essential in the fight against AMR.
These trends highlight the critical role of enteric disease testing in improving public health, enhancing disease management, and responding to the challenges posed by infectious diseases. As the market continues to evolve, innovations in testing technology and global health initiatives will likely drive further growth and development, ensuring that enteric disease testing remains a vital component of healthcare worldwide.
Report Scope
The report analyzes the Enteric Disease Testing market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Testing Method (Conventional Testing, Molecular Diagnostic Testing, Other Testing Methods); Disease Indication (Bacterial Enteric Disease, Parasitic Enteric Disease, Viral Enteric Disease).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Bacterial Enteric Disease Testing segment, which is expected to reach US$1.8 Billion by 2030 with a CAGR of 4.8%. The Parasitic Enteric Disease Testing segment is also set to grow at 4.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $607.5 Million in 2024, and China, forecasted to grow at an impressive 7.4% CAGR to reach $676.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Enteric Disease Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Enteric Disease Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Enteric Disease Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Apollo Diagnostics, bioMerieux Sweden AB, Centre for Infectious Disease Research in Zambia (CIDRZ), EntroGen, Inc., Genovate Laboratories Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 12 companies featured in this Enteric Disease Testing market report include:
- Apollo Diagnostics
- bioMerieux Sweden AB
- Centre for Infectious Disease Research in Zambia (CIDRZ)
- EntroGen, Inc.
- Genovate Laboratories Inc.
- Quest Diagnostics, Inc.
- TECHLAB, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Apollo Diagnostics
- bioMerieux Sweden AB
- Centre for Infectious Disease Research in Zambia (CIDRZ)
- EntroGen, Inc.
- Genovate Laboratories Inc.
- Quest Diagnostics, Inc.
- TECHLAB, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 158 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
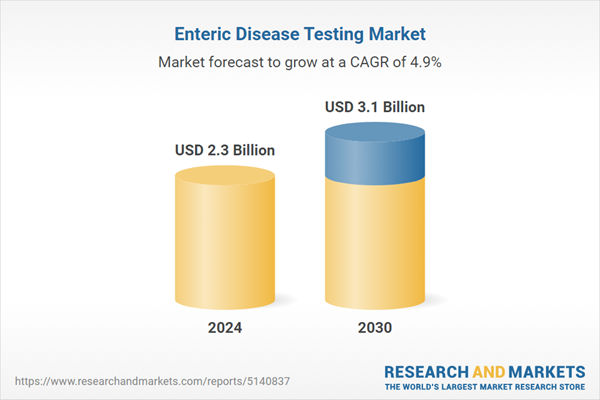
| Estimated Market Value ( USD | $ 2.3 Billion |
| Forecasted Market Value ( USD | $ 3.1 Billion |
| Compound Annual Growth Rate | 4.9% |
| Regions Covered | Global |