Global Influenza Diagnostics Market - Key Trends & Drivers Summarized
What Are the Current Technologies in Influenza Diagnostics?
Influenza diagnostics have become critical for effective healthcare delivery, especially in managing seasonal outbreaks and potential pandemics. Current technologies in influenza diagnostics include rapid influenza diagnostic tests (RIDTs), reverse transcription-polymerase chain reaction (RT-PCR), and viral cultures. RIDTs offer the advantage of speed, providing results in less than 30 minutes, which facilitates swift clinical decisions. However, RT-PCR is the gold standard for influenza diagnostics due to its high sensitivity and specificity, capable of distinguishing between different influenza strains. Viral culture, while less common due to its longer turnaround time, remains an important tool for epidemiological studies and vaccine development. Recent advancements also include the integration of multiplex assays that can detect influenza alongside other respiratory pathogens, enhancing the efficiency and comprehensiveness of testing.How Are Patient Demands Transforming Influenza Diagnostics?
As healthcare consumers become more informed, there is a growing demand for quicker, more accurate diagnostic methods that can be accessed conveniently. This shift is driving the development of point-of-care (POC) tests that can be administered in various settings outside the traditional laboratory environment, such as pharmacies or at home. These tests are designed to be user-friendly, requiring minimal technical skills and providing rapid results to reduce the waiting period and accelerate the initiation of appropriate treatments. This patient-centric approach not only improves individual health outcomes but also aids in controlling the spread of the virus in community settings, highlighting the importance of accessibility and speed in influenza diagnostics.What Impact Does Technology Have on the Evolution of Influenza Diagnostics?
Technological innovation is a key driver in the evolution of influenza diagnostics. The emergence of digital health technologies, including telehealth and mobile health apps, is playing a crucial role in the management of influenza. These technologies allow for remote diagnosis and monitoring, which is particularly beneficial during peak flu seasons or pandemics when healthcare facilities are overwhelmed. Additionally, advancements in genetic sequencing and bioinformatics have improved the accuracy and speed of strain identification, which is vital for effective treatment and vaccination strategies. The ongoing development of biosensors and wearable devices that can detect early symptoms of influenza represents another promising frontier, potentially enabling even earlier detection and intervention.Growth in the Influenza Diagnostics Market Is Driven by Several Factors…
The growth in the influenza diagnostics market is driven by several factors, including the increased global incidence of seasonal influenza and heightened awareness of pandemic threats. Rapid urbanization and international travel contribute to the faster spread of the virus, necessitating robust diagnostic solutions globally. Advances in diagnostic technologies, particularly in rapid testing and genetic analysis, have significantly improved the response time and accuracy of influenza diagnostics. Government initiatives and funding for infectious disease control further support the expansion of the diagnostics market. Additionally, the integration of artificial intelligence and machine learning in diagnostic processes enhances data analysis and interpretation, leading to more precise and predictive diagnostics. As healthcare systems worldwide prioritize preventive care and early intervention, the demand for advanced, rapid influenza diagnostic solutions continues to rise, ensuring sustained growth in this sector.Report Scope
The report analyzes the Influenza Diagnostics market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Test Type (RIDT, RT-PCR, Cell Culture, Other Test Types); End-Use (Hospitals, POCT, Laboratories).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the RIDT segment, which is expected to reach US$2 Billion by 2030 with a CAGR of 6.1%. The RT-PCR segment is also set to grow at 5.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $947.6 Million in 2024, and China, forecasted to grow at an impressive 8% CAGR to reach $1.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Influenza Diagnostics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Influenza Diagnostics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Influenza Diagnostics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alere, Inc., Analytik Jena AG, Becton, Dickinson and Company, DiaSorin SpA, F. Hoffmann-La Roche AG and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 38 companies featured in this Influenza Diagnostics market report include:
- Alere, Inc.
- Analytik Jena AG
- Becton, Dickinson and Company
- DiaSorin SpA
- F. Hoffmann-La Roche AG
- Luminex Corporation
- Meridian Bioscience, Inc.
- Quidel Corporation
- SA Scientific, Ltd.
- Thermo Fisher Scientific, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alere, Inc.
- Analytik Jena AG
- Becton, Dickinson and Company
- DiaSorin SpA
- F. Hoffmann-La Roche AG
- Luminex Corporation
- Meridian Bioscience, Inc.
- Quidel Corporation
- SA Scientific, Ltd.
- Thermo Fisher Scientific, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 171 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
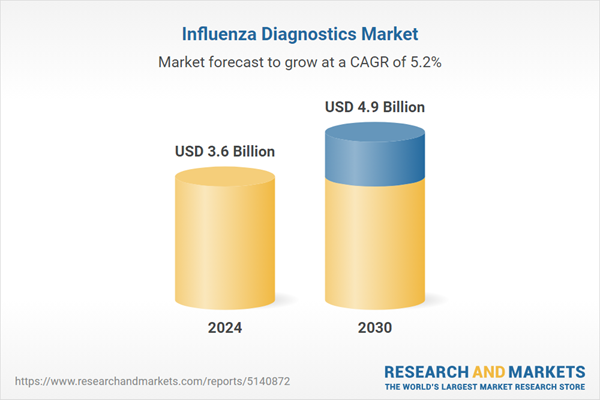
| Estimated Market Value ( USD | $ 3.6 Billion |
| Forecasted Market Value ( USD | $ 4.9 Billion |
| Compound Annual Growth Rate | 5.2% |
| Regions Covered | Global |