The impact of COVID-19 was severe on the studied market owing to the cancellations of elective procedures, including implants of artificial organs, in the Asia Pacific region. However, the market started to gain traction in the last year as transplants and implant procedures resumed in the region. Also, the piling up of elective procedures involving artificial organs and bionics is projected to augment the market during the forecast period.
In addition, the increased incidence of disabilities, the rising number of road accidents, and technological advancements in products leading to enhanced applications are positively affecting the growth of the studied market.
As the number of disabled people grows, so does the demand for bionic implants, which are prosthetics with both mechanical and robotic elements that replace broken body parts such as hands, legs, limbs, and so on.
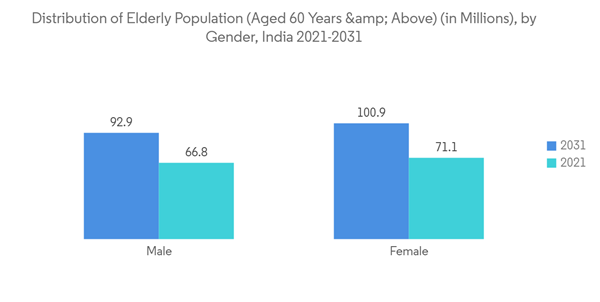
As per the Road Accidents in India report, the number of traffic accidents in the country has increased from 3,68,828 in 2020 to 4,22,659 in 2021. The region's high rate of road accidents, as well as rising injury and trauma, is driving market growth.Furthermore, the growing geriatric population, which is more prone to organ failure, is expected to drive growth during the forecast period.According to the United Nations ESCAP, the Asia-Pacific population is aging faster than any other region in the world. In the current year, there are 630 million people aged 60 and up, accounting for 60% of the world's elderly.By 2050, their number is projected to increase to 1.3 billion. The increase in the trend of the population will help boost the overall market growth.
Various groups of national universities, hospitals, and companies are engaged in a collaborative research and development program to develop artificial organs, which is also funded by the government authorities and the businesses. For example, as part of the Australian federal government's budget announcement in May 2021, Monash University researchers secured Medical Research Future Fund (MRFF) research funding in areas such as maternal health, rare cancers and diseases, silicosis, and nutrition and well-being in remote communities, with the Artificial Heart Frontiers Program receiving USD 999,570 in 2021 for the development of artificial hearts.BiVACOR is also funding and collaborating on the initiative to develop and market a total artificial heart.Such partnerships and research developments are anticipated to boost market growth during the forecast period.
Therefore, owing to the aforementioned factors, it is anticipated that the studied market will witness growth over the analysis period. However, the expensive procedures and fear of device malfunction and its consequences are likely to impede market growth.
APAC Artificial Organs & Bionic Implants Market Trends
Artificial Heart Segment is Expected to Hold a Significant Market Share Over the Forecast Period
The number of people with end-stage heart failure waiting for a heart transplant exceeds the number of available hearts due to the rising burden of cardiovascular disease and congestive heart failure. This has led to an exponential rise in the usage of mechanical circulatory assistance, such as the left ventricular assist device (LVAD) or an artificial heart.Around 1.2 million Australians have one or more heart or vascular diseases in the current year. In September 2021, a national strategic action plan for Heart Disease and Stroke Prevention was created by the Heart Foundation and the Stroke Foundation with support from the Australian government. Heart disease and stroke research are supported by the Medical Research Future Fund and the National Health and Medical Research Council, which have provided USD 220 million over the next few years for the cardiovascular health mission. Such initiatives are projected to boost the research and development activities to develop artificial hearts for patients with heart failure symptoms.
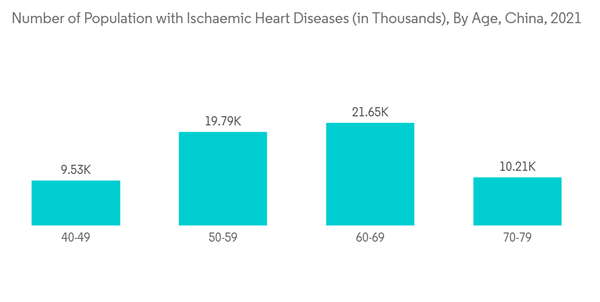
Additionally, the advancements in technology and increasing product approvals, along with partnerships and acquisitions by key players, are helping in the market's growth. For instance, in November 2021, CH Biomedical received marketing approval from the NMPA for its implantable CH-VAD left ventricular assist system. CH-VAD LVAD is one of the first Left Ventricular Assist Devices (LVAD) with completely independent intellectual property rights approved by NMPA in China, and the first complete magnetic levitation LVAD approved by the National Medical Products Administration (NMPA). Such milestones are projected to augment the commercialization of the new generation LVAD in China, offering heart failure treatment, and thereby boosting the market's growth. During the forecast period, the approvals and launches of artificial hearts are anticipated to boost the utility of such devices in the region.
India is Expected to Hold a Significant Share in Asia-Pacific Artificial Organs & Bionic Implants Market Over The Forecast Period
India is expected to hold a significant market share owing to factors such as the rising incidence of disabilities and the rising number of road accidents, the growing geriatric population, and the rising technological advancements and research projects in the country.Various healthcare institutions are engaged in research and development programs to develop artificial organs in the country to reduce expenses so that more people can afford them. For instance, in January 2022, the School of Medical Research and Technology (SMRT) of IIT Kanpur launched Hridyantra, a challenge-based program in collaboration with the country's other hospitals to develop an advanced artificial heart called the Left Ventricular Assist Device (LVAD) for patients with end-stage heart failure. Such research initiatives are projected to expedite more research activities to develop potential artificial organs, thereby boosting market growth in the country.
Furthermore, major manufacturers have focused on developing specialized dialysis equipment coupled with growing partnerships and collaboration among market players, which is expected to drive the artificial kidney market. Currently, various home and in-center hemodialysis machines are available in the market. For instance, in June 2021, Max Healthcare's Max@Home service launched home hemodialysis services for patients in collaboration with NephroPlus. Such developments are anticipated to boost the utility of artificial kidneys, thereby propelling market growth in the country during the forecast period.
APAC Artificial Organs & Bionic Implants Industry Overview
The Asia-Pacific Artificial Organs & Bionic Implants market is slightly consolidated due to the presence of a few companies operating globally as well as regionally. The competitive landscape includes an analysis of a few international as well as local companies that hold significant market shares. Some of the key players are Ossur, Boston Scientific Corporation, Otto Bock Holding GmbH & Co. KG, Medtronic plc, Zimmer Biomet, Edwards Lifesciences Corporation, Sonova Holding AG, Abbott (St. Jude Medical, Inc.), and BiVACOR Inc.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Ossur
- Boston Scientific Corporation
- Otto Bock Holding GmbH & Co. KG
- Medtronic plc
- Zimmer Biomet
- Edwards Lifesciences Corporation
- Sonova Holding AG
- BiVACOR Inc.
- Abbott (St. Jude Medical, Inc.)