The COVID-19 pandemic affected the studied market significantly. Since the beginning of the pandemic, healthcare systems in China resulted in the interruption of usual care in many healthcare facilities, exposing vulnerable patients with cardiovascular diseases to significant risks. However, the demand for cardiovascular devices increased during the pandemic owing to the increased risk of infection among patients with cardiovascular diseases (CVDs). Additionally, research related to CVDs increased in the country during the pandemic. The article 'Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin' published in January 2021 showed that when a case study was conducted on a Chinese male admitted to hospital with chest pain and dyspnoea, chest computed tomography (CT) examination indicated pulmonary infection, enlarged heart, and pleural effusion, while the electrocardiogram (ECG) suspected acute myocardial infarction. Therefore, the diagnostic devices used for diagnoses increased during the pandemic phase. Owing to the burden of heart diseases in the country the adoption of cardiovascular devices used for diagnosis and treatment increased. Thus, the pandemic is expected to have a significant impact on the studied market.
Factors such as rapid technological advances, growing public awareness, and government initiatives related to cardiovascular diseases are expected to fuel cardiovascular devices market growth in China.
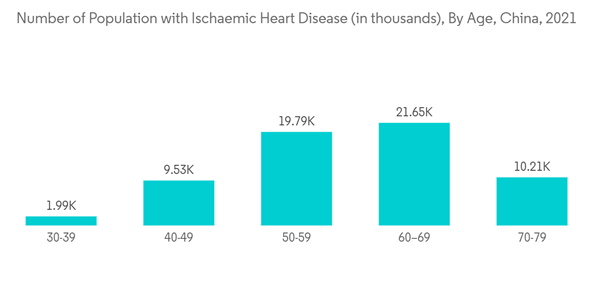
Cardiovascular disease (CVD) is the leading cause of healthcare burden in China. The prevalence and incidence of the disease are increasing with time. The research article 'Cardiovascular Disease Mortality and Potential Risk Factor in China: A Multi-Dimensional Assessment by a Grey Relational Approach' published in April 2022 mentioned that CVD is one of the major healthcare burdens in China. And as per the article, currently, 290 million people suffering from cardiac disorders (13 million), Stroke, coronary heart disease (11 million), rheumatic heart disease (2.5 million), heart failure (4.5), congenital heart disease (2 million), pulmonary heart disease (5 million), and hypertension (245 million) in China. Also, the source above, mentioned that out of the total mortality related to CVD, 40% occurs in China alone. Thus, such burden and persistent risk of CVD among the target population in China is expected to demand cardiology medical devices for treatment and diagnosis. Thereby, driving the market growth.
Furthermore, the major or minor companies present in China are focused on developing advanced technologies for the treatment of the high-burden target population. This is one of the prominent factors that is expediating the cardiovascular devices market growth in China. For instance, in January 2022, Medtronic plc, a global healthcare technology received approval from the National Medical Products Administration (NMPA) for its CoreValve Evolut PRO TAVR system for the treatment of severe aortic stenosis (AS) for symptomatic patients in China who are at high or extreme risk for open heart surgery.
In addition, a joint venture between Genesis MedTech and Shockwave was formed in March of 2021 to distribute Shockwave's products in China and to strengthen Genesis MedTech's Wuxi research and development and production base to accelerate the manufacturing of Shockwave's products for the local Chinese market. Further in May 2022, Shockwave Medical, Inc. and Genesis MedTech Group successfully obtained approval from China's National Medical Products Administration (NMPA) to market and sell the Shockwave IVL System with the Shockwave C2 Coronary IVL Catheters and the Shockwave M5 and S4 Peripheral IVL Catheters in China. Such developments in the country are expected to drive the studied market growth during the analysis period.
Therefore, the aforesaid factors are expected to contribute to the growth of the cardiovascular devices market in China. However, stringent regulatory systems for several products might hamper the market growth over the analysis period.
Cardiovascular Devices Market Trends
Stents by Therapeutic and Surgical Devices Sub-Segment is Expected to Witness Growth Over the Forecast Period
A stent, which resembles a tiny coil of wire mesh, supports the walls of the artery and aids in preventing it from re-narrowing is used during angioplasty. These are currently used most frequently in most patients undergoing angioplasty. Once implanted, stents are designed to be permanent and will keep patients' arteries open. Thus, the stent by therapeutic and surgical devices segment is anticipated to gain demand and witness growth over the analysis period.The government of China is taking initiatives to increase the production of medical devices in the country and in 2020 the volume-based procurement (VBP) act was announced. The article 'Volume-based procurement is shaking up high-value medical devices market in China' published in January 2022 stated that VBP of high-value medical supplies, beginning with coronary stents, has been practiced in China to combat the increased costs of high-value medical products. Thus, such an initiative by the government of China is expected to create opportunity for the stent segment. This is expected to drive the segment growth.
Additionally, frequent product launches and advancements are other major factors that are expected to fuel market growth. For instance, in November 2021, Biosensors International, a developer, and manufacturer of innovative medical devices declared positive results from the BIO-RISE CHINA, a multi-center, randomized controlled trial performed in 10 centers across China and this first-in-human study assessed the safety and efficacy of a Biolimus A9 (BA9) coated balloon (DCB) used in both BioMatrix and BioFreedom families of coronary stents, in patients with small vessel coronary artery disease undergoing percutaneous coronary intervention (PCI).
In addition, in August 2021, SINOMED, an international medical device company in China, declared the first commercial implantation of the HT Supreme Drug-Eluting Stent (DES) at University Hospital Galway in partnership with the National University of Ireland Galway (NUI Galway). Thus, such advancements are anticipated to drive segment growth in the future.
Therefore, the above-mentioned factors are expected to drive the segment growth significantly and contribute to the overall market growth over the forecast period.
Cardiovascular Devices Industry Overview
The competitive landscape of the China Cardiovascular devices market covers the business overview, financials, products, and strategies followed by major companies. The cardiovascular devices market in China is highly competitive and consists of several major players. In terms of market share, a few of the major players are currently dominating the market. Some of the Key companies which are currently dominating the market are Abbott Laboratories, Boston Scientific Corporation, Mindray Medical International Limited, Edwards Lifesciences, WL Gore & Associates Inc., Medtronic PLC, Lepu Medical Technology (Beijing) Co. Ltd, Terumo Corporation, and Microport Scientific Corporation, among others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Boston Scientific Corporation
- Mindray Medical International Limited
- Edwards Lifesciences
- WL Gore & Associates Inc.
- Medtronic PLC
- Lepu Medical Technology (Beijing) Co. Ltd
- Terumo Corporation
- Microport Scientific Corporation
- Suzhou Haobro Medical Device Co.,Ltd
- Kossel Medtech (Suzhou) Co., Ltd.
- Elite Medtek(Jiangsu) Co., Ltd
- Insight Lifetech Co., Ltd.