Biosimilars applications span numerous therapeutic areas, including oncology (for example, trastuzumab in cancer therapy), blood disorders (such as epoetin for treating anemia), autoimmune conditions (like infliximab for rheumatoid arthritis), and deficiencies in growth hormones. Additional applications include managing chronic conditions such as diabetes, enhancing immune defenses against infectious diseases, and treating cardiovascular and other disorders requiring immune system regulation. Following the expiration of the reference product's patent, biosimilars undergo stringent evaluations by regulatory authorities such as the FDA, EMA, and Health Canada to ensure they adhere to strict standards for quality, safety, and effectiveness.
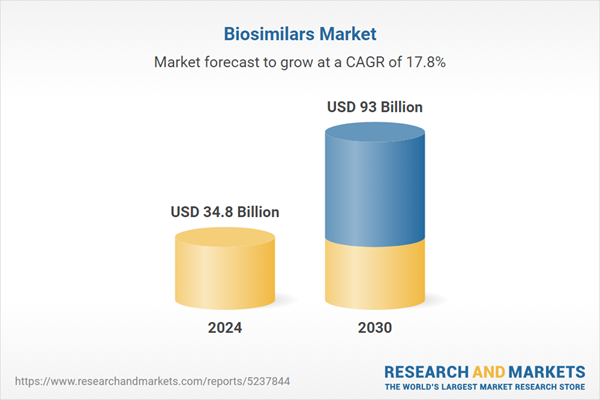
The global biosimilars market is estimated at a value of US$34.8 billion in 2024 and projected to reach US$93.1 billion by 2030, exhibiting a CAGR of 17.8% from 2024 to 2030 driven by the increasing cost of biologic drugs, leading to a greater demand for more affordable options. Biosimilars provide cost-effective alternatives while delivering comparable therapeutic results, making them a desirable choice for patients and healthcare systems. The biologics patent expiration is another significant driver, allowing biosimilar manufacturers to offer affordable options while maintaining similar therapeutic efficacy. The rising incidence of chronic conditions, including cancer, kidney disease, arthritis, and Crohn's disease, further enhances the demand for biosimilars, which are utilized in managing these ailments. Moreover, the increasing expenditure on healthcare and the need to cut drug costs have prompted governments and insurers to endorse biosimilars. The growth of approval rates and regulatory measures in major markets such as the US, EU, India, China, and Brazil also facilitates the development of biosimilars, contributing to market growth. With increased awareness of biosimilars and their advantages, alongside rising R&D investments, the market is projected to grow substantially in the coming years.
Biosimilars Regional Market Analysis
Europe dominates the global biosimilars market with an estimated 40.8% share in 2024 due to its robust healthcare system, a rising prevalence of diseases, and a supportive regulatory framework. The region's swift adoption and increased awareness of biosimilars among healthcare professionals and patients play a crucial role in market expansion. The competitive pricing of biosimilars has resulted in significant cost reductions, further enhancing their adoption in this region. Conversely, the Asia-Pacific region is experiencing rapid growth, with a projected CAGR of 20.3% during the forecast period 2024-2030. This growth is driven by increased investments in pharmaceutical research and development, a rising demand for affordable treatments, and an aging population in countries such as China, India, and Japan. The market in this region, particularly in China and India, is expanding due to improved disposable incomes and an increasing incidence of chronic illnesses like cancer, diabetes, and autoimmune diseases.Biosimilars Market Analysis by Product Type
The monoclonal antibodies (moAbs or mAbs) segment led the biosimilars market in 2024, capturing a 56% share due to their widespread application in treating conditions such as cancer, rheumatoid arthritis, cardiovascular issues, and multiple sclerosis. The expiration of patents for major moAbs, such as Trastuzumab and Adalimumab, has resulted in increased adoption of moAb biosimilars as more affordable alternatives. On the other hand, the erythropoietin (EPO) segment is anticipated to record the fastest growth with a CAGR of 19.2% during the analysis period 2024-2030. The increasing use of EPO biosimilars to manage anemia in patients with chronic kidney disease and those undergoing oncology treatment, along with a rise in regulatory approvals and financial pressures, is fueling their market growth.Biosimilars Market Analysis by Therapeutic Area
The oncology application is the largest segment in the biosimilars market in 2024, with an estimated 42.6% share, and is anticipated to experience the fastest growth with a CAGR of 18.4% from 2024 to 2030, spurred by the increasing global cancer rates. The growing use of biosimilars in treating cancers like breast, colorectal, and solid tumors is improving patient survival and quality of life. The expiration of patents for key biologics has accelerated the adoption of biosimilar monoclonal antibodies, and the cost benefits of biosimilars are further fueling growth. The autoimmune diseases segment, the second largest with a 22.6% share in 2024, is growing due to the rising prevalence of conditions like rheumatoid arthritis and psoriasis. Biosimilars offer more affordable alternatives to costly biologics, increasing treatment accessibility. As regulatory approvals and acceptance continue to rise, this segment is set for substantial growth driven by the increasing demand for treatments for chronic autoimmune disorders.Biosimilars Market Report Scope
This global report on Biosimilars analyzes the global and regional markets based on product type and therapeutic area for the period 2024-2030 with projections from 2024 to 2030 in terms of value in US$. In addition to providing profiles of major companies operating in this space, the latest corporate and industrial developments have been covered to offer a clear panorama of how and where the market is progressing.Key Metrics
- Historical Period: 2021-2023
- Base Year: 2024
- Forecast Period: 2024-2030
- Units: Value market in US$
- Companies Mentioned: 30+
Biosimilars Market by Geographic Region
- North America (The United States, Canada, and Mexico)
- Europe (Germany, France, United Kingdom, Italy, Spain, and Rest of Europe)
- Asia-Pacific (Japan, China, India, South Korea, and Rest of Asia-Pacific)
- Rest of World (Brazil, Israel, and Other Countries)
Biosimilars Market by Product Type
- Monoclonal Antibodies (mAbs)
- Erythropoietin (EPO)
- Granulocyte Colony-Stimulating Factor (G-CSF)
- Insulin
- Others (Including Interferons, Follitropins, Recombinant Proteins, and Others)
Biosimilars Market by Therapeutic Area
- Oncology
- Autoimmune Disorders
- Blood Disorders
- Growth Hormone Deficiency
- Other Therapeutic Areas (Including Chronic Diseases, Infectious Diseases, Cardiovascular Disorders, and Others)
This product will be delivered within 2-4 business days.
Table of Contents
Companies Mentioned
- AbbVie Inc.
- Amgen Inc.
- BIOCAD
- Biocon Ltd.
- Biogen Inc.
- Bioton S.A.
- Boehringer Ingelheim International GmbH
- Celltrion Inc.
- Coherus BioSciences, Inc.
- Dr. Reddy's Laboratories Ltd.
- Eli Lilly and Company
- F. Hoffmann-La Roche AG
- Fresenius Kabi AG
- Genor Biopharma Co. Ltd
- Hetero Drugs Limited
- Intas Pharmaceuticals Ltd.
- Kashiv BioSciences, LLC
- LG Chem
- Lupin Limited
- mAbxience
- Merck KGaA
- Nippon Kayaku Co., Ltd
- Novartis AG
- Pfizer Inc.
- Reliance Life Sciences Pvt. Ltd.
- Samsungbioepis Co. Ltd
- Sandoz Group AG
- Shanghai Henlius Biotech, Inc.
- STADA Arzneimittel AG
- STgen Bio Co., Ltd.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- Wockhardt Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 227 |
| Published | February 2025 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 34.8 Billion |
| Forecasted Market Value ( USD | $ 93 Billion |
| Compound Annual Growth Rate | 17.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 33 |