The term"fill/finish describes the final steps of the manufacturing process, which are filling and finishing. Filling in the pharmaceutical industry means putting medication into a container and closing it, whereas finishing refers to sterilizing and standardizing medical supplies and containers.
Growing Adoption of Prefilled Syringes for Parenteral Administration drives the growth of the Fill Finish Manufacturing Market.
Parenteral administration is one of the most prominent routes chosen to stimulate immediate immune response and ensure the complete bioavailability of pharmaceutical products. A steady rise in the development and market availability of parenteral drugs has propelled the demand for advanced, cost-effective drug delivery devices that promise ease of administration. The benefits of prefilled syringes over traditional delivery systems include easy administration, improved safety, accurate dosing, and reduced contamination risks. Among drug delivery devices, prefilled syringes represent one of the fastest-growing primary packaging formats, which are designed for dose administration. In the past ten years, there has been an evident increase in the development of parenteral drugs (especially with the introduction of several classes of biologics), which has resulted in approximately three-fold increase in the consumption of prefilled syringes. The sustained preference for the prefilled syringes is attributed to the safety and ease of use of these products. Recent variants are designed with provisions to reduce errors in dosing, risk of occlusions, leakage of fluids (i.e., extravasation), and inflammation of veins (phlebitis). Owing to the benefits mentioned above, several injectable drugs - Humira, Enbrel, Avastin, PREVNAR 13, ALPROLIX, and Benefix, among others - diluents and other products requiring parenteral administration are packaged in prefilled syringes.
Over the past seven years, ~90 drugs have been approved in the prefilled syringe packaging form across different geographies, including North America, Europe, and Asia Pacific. Several drugs in the clinical stages of drug development are being evaluated in combination with prefilled syringes.
The loading of sterile drugs into prefilled syringes is considered one of the most crucial steps in the pharmaceutical production process. Proper fill-finish operations are necessarily carried out under aseptic conditions to maintain pharmacological efficacy and quality and to ensure the safety of end users. The prefilled syringe filling is a complex operation as it requires extremely close monitoring of both the syringe fill volume and the headspace between the liquid filled in the syringe and the bottom of the plunger. In addition, the rise in complexity of small molecule APIs and the increasing diversity of biological drugs contribute to the demand for advanced aseptic fill finish operations.
Companies, including small enterprises and large businesses, outsource their respective fill finish operations to contract service providers. Per the 10th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, manufacturers of biological have been observed to outsource more than 30% of their fill finish operations. With the rise in the demand for prefilled syringes and the growing complexity of fill finish processes, outsourcing these operations is likely to increase in the future. Over 100 companies across the globe are providing fill finish services for prefilled syringe manufacturers. To cater to the growing demand for pharmaceutical products, service providers are actively investing in expanding their existing infrastructure and capabilities; they have also expanded their clientele through service agreements over the past few years. As injectables account for ~55% of drug candidates in the global R&D pipeline, the businesses of prefilled syringe manufacturers and associated service providers are also growing. Due to the emergence of the COVID-19 crisis, vaccine development initiatives have increased across the globe, which significantly boosted the demand for prefilled syringes. Thus, the rising adoption of prefilled syringes for parenteral administration drives the fill finish manufacturing market.
The fill finish manufacturing market is divided on the basis of product, modality, and end user. Based on product, the fill finish manufacturing market is segmented into consumables and instruments. The consumables segment is further divided into prefilled syringes, glass vials/plastic vials, cartridges, and others. By modality, the fill finish manufacturing market is segmented into recombinant proteins, monoclonal antibodies, vaccines, cell therapies and biological therapies, gene therapies, and others. In terms of end users, the fill finish manufacturing market is classified as contract manufacturing organizations, biopharmaceutical companies, and others.
Based on geography, the fill finish manufacturing market is divided into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. North America is the most significant contributor to the growth of the fill finish manufacturing market. Canada is among the fastest-developing countries in terms of the biopharmaceutical industry. Various international companies are investing in the Canadian biopharmaceutical industry. In Canada, the number of biopharmaceutical companies is rising significantly. For instance, Vancouver, British Columbia, has the majority of biopharmaceutical companies, which generate US$ 90.3 million (CAD 120 million) revenue annually. There are ~35-40 domestic biopharmaceutical companies across the country. A few of the Canadian biopharmaceutical companies include Inex Pharmaceuticals, Quest Vitamins, StressGen Biotechnologies, Stanley Pharmaceuticals, QLT Phototherapeutics, and Stemcell Technologies. Various public and private research organizations include the Center for Molecular Medicine and Therapeutics, the Biotechnology Laboratory at the University of British Columbia, the BC Cancer Research Center, the Canadian HIV Trials Network, and the Vaccine Evaluation Center. Thus, the development of the biopharmaceutical industry in the country will bolster the market growth during the forecast period
Asia Pacific is expected to be the fastest-growing market in the coming years. In Asia Pacific, China is the largest market for fill finish manufacturing. The growth of the market is primarily attributed to the rising technological advancements in fill finish manufacturing processes in China, increasing developments by the market players, the biopharmaceutical industry’s expansion, and the growing prevalence of chronic diseases. There are more than 500 biological product/biopharmaceutical companies in China. Most of those involved in R&D were established by returnees from abroad or by Western/joint venture companies. Although estimates vary widely, analysts believe that the Chinese government spends more than US$ 600 million annually on biotech R&D through its funding initiatives. China’s national and local governments also invest in quasi-venture capital companies that invest in IT enterprises. The market players are expanding their business through organic and inorganic growth strategies. For instance, WuXi Biologics increased the capacity of prefilled syringes (PFS) to 17 million units yearly in June 2022 by opening its drug product factory in Wuxi, China. The most recent D.P. facility operated by WuXi Bio, a contract development manufacturing organization (CDMO), is called DP5, and it has an advanced isolator filling line for reliable, continuous filling services. According to the company, this provides PFS with a variety of volume delivery options, including 1.25 mL, 3 mL, 1 mL, and 1 mL.ClinicalTrails.com, Centers for Disease Control and Prevention (CDC), and Food and Drug Administration (FDA) are a few key primary and secondary sources referred to while preparing the report on the fill finish manufacturing market.
Reasons to Buy
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the fill finish manufacturing market.
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the fill finish manufacturing market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth market trends and outlook coupled with the factors driving the fill finish manufacturing market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin security interest with respect to client products, segmentation, pricing, and distribution.
Table of Contents
Companies Mentioned
- IMA Industria Macchine Automatiche SpA
- Nipro Medical Europe NV
- Maquinaria Industrial Dara SL
- Groninger and Co GmbH
- SGD SA
- Optima Packaging Group Gmbh
- NNE AS
- Stevanato Group SpA
- Syntegon Technology GmbH
- West Pharmaceutical Services Inc
- Gerresheimer AG
- Schott AG
- Becton Dickinson and Co
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2023 |
| Forecast Period | 2022 - 2030 |
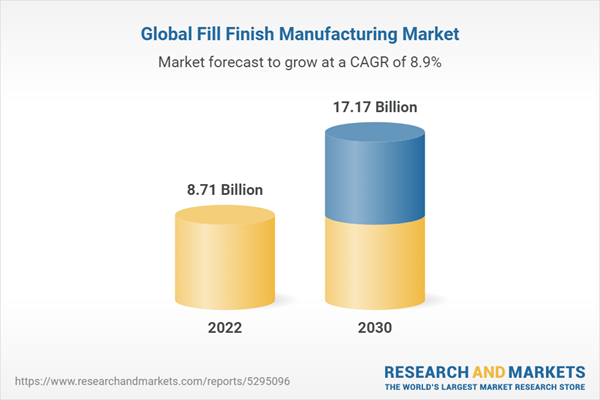
| Estimated Market Value in 2022 | 8.71 Billion |
| Forecasted Market Value by 2030 | 17.17 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |