Global Rapid Antigen Detection Tests Market - Key Trends & Drivers Summarized
What Are Rapid Antigen Detection Tests and How Are They Manufactured?
Rapid antigen detection tests (RADTs) are diagnostic tools designed to quickly identify the presence of specific antigens in a patient's sample, typically from nasal or throat swabs. These tests have gained significant attention for their ability to provide rapid results, often within 15 to 30 minutes, making them particularly valuable in clinical and public health settings for diagnosing infectious diseases, such as COVID-19, influenza, and strep throat. The speed and ease of use of RADTs allow healthcare providers to make timely decisions regarding patient care and treatment, as well as implement appropriate public health measures.The manufacturing process of rapid antigen detection tests involves several steps, including the development of specific antibodies that can bind to the target antigens. These antibodies are typically immobilized on a solid surface, such as a test strip or device, to capture the antigens present in the sample. The test kit usually includes reagents that produce a detectable signal - such as a color change - indicating the presence of the antigen. Quality control measures are implemented throughout the manufacturing process to ensure that the tests meet regulatory standards for sensitivity, specificity, and reliability.
Recent advancements in the production of rapid antigen detection tests focus on improving accuracy, usability, and scalability. Manufacturers are exploring the use of novel materials and technologies to enhance the sensitivity and specificity of tests while reducing false-negative rates. Additionally, there is an ongoing effort to simplify the testing process, making it easier for non-professionals to administer tests in home or community settings. The integration of digital technology for result interpretation and data management is also being explored to enhance the user experience and streamline reporting processes.
What Are the Primary Applications of Rapid Antigen Detection Tests Across Industries?
Rapid antigen detection tests are primarily utilized in the healthcare sector, particularly in clinical laboratories, hospitals, and point-of-care settings. One of the primary applications of RADTs is in the diagnosis of respiratory infections, such as COVID-19 and influenza. During the COVID-19 pandemic, RADTs became crucial for mass testing efforts, enabling healthcare providers to quickly identify infected individuals and implement necessary isolation measures. The ability to deliver rapid results has made RADTs an essential tool in controlling the spread of infectious diseases, especially in high-transmission environments such as schools, workplaces, and healthcare facilities.In addition to respiratory infections, rapid antigen detection tests are also used for diagnosing other infectious diseases, such as streptococcal throat infections and certain gastrointestinal pathogens. These tests provide a convenient and efficient way to identify infections that require prompt treatment, thereby reducing the risk of complications and the spread of illness. The versatility of RADTs across various disease states makes them an integral part of diagnostic testing in clinical practice.
Another significant application of RADTs is in public health surveillance and outbreak management. Public health agencies utilize rapid antigen tests to conduct mass screening initiatives during outbreaks or pandemics, helping to quickly identify and isolate infected individuals. The rapid turnaround time of results allows for timely interventions, such as contact tracing and targeted public health messaging. This application is particularly valuable in managing infectious disease outbreaks in crowded settings, such as nursing homes, schools, and event venues.
Furthermore, the use of rapid antigen detection tests is expanding into at-home testing kits, allowing individuals to self-administer tests for various infections. This shift towards home testing is driven by consumer demand for convenient and accessible healthcare solutions, particularly in the context of the ongoing COVID-19 pandemic. The ability to perform tests at home enhances public participation in health monitoring and disease prevention, contributing to better overall health outcomes.
Why Is Consumer Demand for Rapid Antigen Detection Tests Increasing?
The demand for rapid antigen detection tests is increasing due to several key factors, including the ongoing need for efficient diagnostic solutions, heightened awareness of infectious diseases, and the shift towards at-home healthcare. One of the primary drivers of demand is the continued focus on rapid and accurate diagnostics in the wake of the COVID-19 pandemic. As governments and healthcare providers strive to manage the spread of the virus, rapid antigen tests have become essential tools for testing and surveillance. The ability to quickly identify infected individuals and take appropriate public health measures has heightened the importance of RADTs in healthcare strategies.In addition to COVID-19, there is a growing recognition of the value of rapid antigen tests in diagnosing other infectious diseases. The demand for efficient diagnostic solutions extends to respiratory illnesses like influenza, where rapid testing can facilitate timely treatment and reduce the risk of complications. As healthcare providers seek to enhance their diagnostic capabilities, RADTs are becoming increasingly attractive for both acute and chronic disease management.
The expansion of at-home testing solutions is another significant factor contributing to the rising demand for rapid antigen detection tests. Consumers are increasingly seeking convenient and accessible healthcare options, particularly for monitoring infectious diseases. The availability of rapid antigen tests for home use empowers individuals to take control of their health, allowing for timely testing and peace of mind. This trend has been further accelerated by the COVID-19 pandemic, which has prompted many to adopt regular testing practices as a precautionary measure.
Moreover, advancements in technology and manufacturing processes are making rapid antigen detection tests more reliable and user-friendly. Innovations in test design, including improved sensitivity and specificity, are enhancing the performance of RADTs, increasing consumer confidence in their results. As manufacturers continue to develop and refine rapid testing technologies, the availability of high-quality, accurate tests is expected to drive further demand.
What Factors Are Driving the Growth of the Rapid Antigen Detection Tests Market?
The growth of the rapid antigen detection tests market is driven by several key factors, including the rising prevalence of infectious diseases, advancements in diagnostic technologies, and increasing investments in public health infrastructure. One of the most significant factors influencing market growth is the ongoing demand for effective testing solutions to manage infectious disease outbreaks. The COVID-19 pandemic has underscored the critical role of rapid testing in public health response efforts, leading to substantial investments in the development and distribution of RADTs. As new variants and other infectious diseases emerge, the need for reliable and timely testing solutions will remain a priority for healthcare systems worldwide.Advancements in diagnostic technologies are also playing a crucial role in driving the growth of the rapid antigen detection tests market. Manufacturers are continually improving the sensitivity and specificity of tests while reducing the time required to obtain results. The integration of smart technologies, such as mobile applications for result tracking and reporting, is enhancing the user experience and increasing the adoption of rapid antigen tests in both clinical and home settings. These technological innovations are making RADTs more accessible and appealing to healthcare providers and consumers alike.
The increasing emphasis on public health preparedness and response is another important factor contributing to market growth. Governments and health organizations are recognizing the need for robust testing strategies to prevent and control the spread of infectious diseases. As part of these efforts, rapid antigen detection tests are being integrated into comprehensive testing protocols for schools, workplaces, and community settings. This focus on proactive public health measures is expected to drive sustained demand for RADTs in various applications.
Additionally, the growing trend toward at-home testing is influencing the rapid antigen detection tests market. Consumers are increasingly seeking convenient, user-friendly solutions for self-testing, especially in the context of infectious disease monitoring. The availability of rapid antigen tests for home use is enabling individuals to take charge of their health and engage in preventive measures more effectively. As this trend continues to grow, the demand for at-home RADTs will play a significant role in shaping the market landscape.
In conclusion, the global rapid antigen detection tests market is poised for robust growth, driven by the increasing prevalence of infectious diseases, advancements in diagnostic technologies, and the growing emphasis on public health preparedness. As healthcare providers and consumers seek efficient and effective solutions for managing health and preventing disease spread, rapid antigen tests will remain essential tools in the diagnostic landscape. With ongoing innovations and a commitment to enhancing public health outcomes, the market for rapid antigen detection tests is expected to experience sustained expansion in the coming years.
Report Scope
The report analyzes the Rapid Antigen Detection Tests market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Segment (Rapid Antigen Detection Tests).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Regional Analysis
Gain insights into the U.S. market, valued at $88.4 Million in 2024, and China, forecasted to grow at an impressive 12.4% CAGR to reach $143.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Rapid Antigen Detection Tests Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Rapid Antigen Detection Tests Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Rapid Antigen Detection Tests Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Acumen Research Laboratories, Beijing Tigsun Diagnostics Co. Ltd., Biolidics, Biomaxima and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Rapid Antigen Detection Tests market report include:
- Abbott Laboratories
- Acumen Research Laboratories
- Beijing Tigsun Diagnostics Co. Ltd.
- Biolidics
- Biomaxima
- BioMedomics
- CTK Biotech
- Getein Biotech
- LabCorp
- ThermoFisher
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Acumen Research Laboratories
- Beijing Tigsun Diagnostics Co. Ltd.
- Biolidics
- Biomaxima
- BioMedomics
- CTK Biotech
- Getein Biotech
- LabCorp
- ThermoFisher
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 130 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
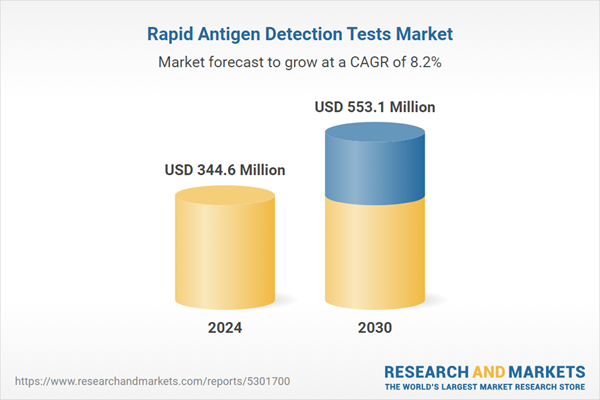
| Estimated Market Value ( USD | $ 344.6 Million |
| Forecasted Market Value ( USD | $ 553.1 Million |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |