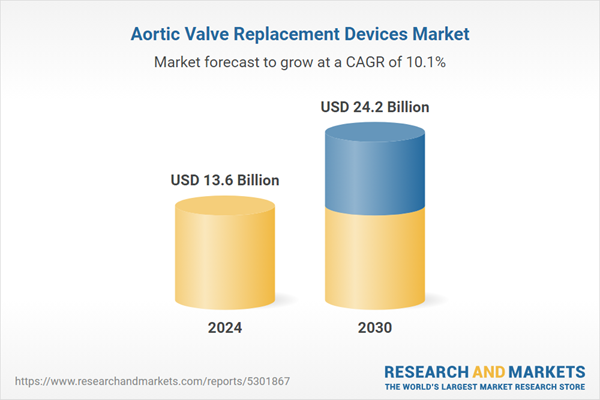
Global Aortic Valve Replacement Devices Market - Key Trends and Drivers Summarized
Why Are Aortic Valve Replacement Devices Critical for Treating Heart Disease?
Aortic valve replacement devices have become essential tools in the treatment of aortic valve diseases, particularly aortic stenosis and aortic regurgitation, but why are these devices so crucial for patients with heart conditions? The aortic valve plays a vital role in the heart's ability to pump blood efficiently to the rest of the body. In patients suffering from aortic valve disease, the valve becomes narrowed (stenosis) or leaks (regurgitation), leading to symptoms such as shortness of breath, chest pain, fatigue, and, in severe cases, heart failure. If left untreated, these conditions can be life-threatening. Aortic valve replacement devices, including both surgical and transcatheter options, offer a life-saving solution by restoring normal blood flow through the heart. These devices come in two primary forms: mechanical valves, which are made from durable materials like titanium and carbon, and biological valves, derived from animal tissue or human donors. While mechanical valves offer long-term durability, biological valves tend to require less anticoagulation therapy, making them a popular choice for certain patient populations. As heart disease remains one of the leading causes of death worldwide, aortic valve replacement has become a standard and critical intervention for improving the quality of life and survival rates in affected individuals.What Advances in Technology Are Shaping the Future of Aortic Valve Replacement?
The field of aortic valve replacement has seen significant technological advancements, particularly with the development of less invasive procedures like transcatheter aortic valve replacement (TAVR). Traditionally, aortic valve replacement required open-heart surgery, which, while effective, carried significant risks, especially for older or high-risk patients. The advent of TAVR has revolutionized the treatment of aortic valve disease by providing a minimally invasive alternative. In this procedure, a replacement valve is delivered via a catheter through a blood vessel in the leg or chest, eliminating the need for open-heart surgery. TAVR has opened up treatment options for patients who were previously considered inoperable due to age or other health conditions. Recent advancements in TAVR technology have also improved the precision and durability of these devices, reducing complications like valve leakage (paravalvular leak) and improving long-term outcomes. In addition to TAVR, there have been significant innovations in valve design. New-generation valves are now more durable, and many feature advanced coatings or materials that reduce the risk of calcification and clot formation, common complications in earlier models. Research into polymer and bioresorbable valves is ongoing, with the aim of creating devices that mimic natural tissue more closely and reduce the need for lifelong anticoagulation therapy. Additionally, the integration of imaging technologies like 3D echocardiography and advanced fluoroscopy has enhanced the accuracy of valve placement, further improving patient outcomes.How Are Aortic Valve Replacement Devices Improving Patient Outcomes?
Aortic valve replacement devices are dramatically improving patient outcomes, not only by extending life expectancy but also by enhancing quality of life. Patients with severe aortic stenosis or regurgitation who undergo valve replacement typically experience immediate relief from symptoms such as shortness of breath, fatigue, and chest pain, allowing them to return to daily activities and enjoy a more active lifestyle. Furthermore, studies have shown that valve replacement significantly reduces the risk of heart failure and other cardiovascular complications, leading to a marked improvement in survival rates. The introduction of transcatheter techniques like TAVR has been particularly impactful for elderly or high-risk patients, who often face a higher risk of complications with traditional open-heart surgery. TAVR offers a shorter recovery time, reduced hospital stays, and a lower risk of complications such as infections or arrhythmias. In younger or more active patients, mechanical valves have provided a long-term solution due to their durability, often lasting decades without the need for replacement. However, these patients typically require lifelong anticoagulation therapy to prevent blood clots, which can pose a risk of bleeding. On the other hand, biological valves, while having a shorter lifespan (10-20 years), are often recommended for older patients or those who prefer to avoid the risks associated with anticoagulation therapy. With advances in materials and design, new biological valves are becoming more durable, extending the time between valve replacements. Overall, the improvement in valve technology and surgical techniques has led to better post-operative outcomes, fewer complications, and an enhanced quality of life for patients who undergo aortic valve replacement.What's Fueling the Expansion of the Aortic Valve Replacement Devices Market?
The growth in the aortic valve replacement devices market is driven by several factors, including the rising prevalence of aortic valve disease, aging populations, and advancements in minimally invasive procedures. One of the primary drivers is the increasing incidence of aortic stenosis, particularly among older adults. As the global population ages, the number of individuals suffering from aortic valve disease continues to rise, creating a growing demand for effective treatment solutions. This demographic shift, combined with better diagnostic tools that allow earlier detection of valve disease, is expanding the patient pool eligible for valve replacement. Additionally, the success and rapid adoption of transcatheter aortic valve replacement (TAVR) procedures have fueled market growth. Initially approved only for high-risk patients, TAVR is now increasingly being used for intermediate - and low-risk patients, significantly broadening its application. Another key factor is the advancement in valve technology and materials. Innovations in biocompatible materials, improved valve designs, and enhanced durability have made both mechanical and biological valves more effective and longer-lasting. The development of minimally invasive techniques, such as TAVR, has also reduced the recovery time and risk associated with valve replacement, making the procedure more appealing to both patients and healthcare providers. Furthermore, the increasing awareness of heart health, driven by public health campaigns and medical advancements, has encouraged more patients to seek treatment for aortic valve conditions earlier, further driving market demand. The rise in healthcare spending and improvements in medical infrastructure, particularly in emerging markets, are also contributing to the growth of the aortic valve replacement devices market. As healthcare systems invest in advanced cardiovascular care, the demand for these life-saving devices is expected to continue expanding, offering new opportunities for innovation and improved patient outcomes.Report Scope
The report analyzes the Aortic Valve Replacement Devices market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Surgery (Minimally Invasive Surgery, Open Surgery); Product (Transcatheter Aortic Valve, Sutureless Valve, Other Products); End-Use (Hospitals, Ambulatory Surgery Centers, Other End-Uses).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Minimally Invasive Surgery segment, which is expected to reach US$15.2 Billion by 2030 with a CAGR of a 10.9%. The Open Surgery segment is also set to grow at 8.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.5 Billion in 2024, and China, forecasted to grow at an impressive 13.6% CAGR to reach $5.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Aortic Valve Replacement Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Aortic Valve Replacement Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Aortic Valve Replacement Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott, BIOTRONIK, Inc., Boston Scientific Corporation, Cryolife, Inc., Edwards Lifesciences Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Aortic Valve Replacement Devices market report include:
- Abbott
- BIOTRONIK, Inc.
- Boston Scientific Corporation
- Cryolife, Inc.
- Edwards Lifesciences Corporation
- HLT
- JC Medical, Inc.
- JenaValve Technology, Inc.
- LivaNova PLC. (Sorin Group)
- Medtronic Plc
- St. Jude Medical
- Symetis SA
- Venus Medtech
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott
- BIOTRONIK, Inc.
- Boston Scientific Corporation
- Cryolife, Inc.
- Edwards Lifesciences Corporation
- HLT
- JC Medical, Inc.
- JenaValve Technology, Inc.
- LivaNova PLC. (Sorin Group)
- Medtronic Plc
- St. Jude Medical
- Symetis SA
- Venus Medtech
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 372 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 13.6 Billion |
| Forecasted Market Value ( USD | $ 24.2 Billion |
| Compound Annual Growth Rate | 10.1% |
| Regions Covered | Global |