Global Fusion Proteins Market - Key Trends & Drivers Summarized
What Are Fusion Proteins & Why Are They Essential in Biopharmaceuticals?
Fusion proteins are engineered proteins created by merging two or more distinct genes into a single gene, resulting in a hybrid protein with combined functions of its original components. These proteins are designed to enhance therapeutic efficacy, target specificity, and stability in various medical treatments. Fusion proteins are widely used in biopharmaceuticals, serving as therapeutic agents in treating cancer, autoimmune diseases, infectious diseases, and rare genetic disorders. They are also employed in drug delivery, vaccine development, and diagnostic applications, where their unique properties enable improved targeting, longer half-life, and enhanced bioavailability.The demand for fusion proteins has grown significantly due to their ability to address complex diseases with enhanced therapeutic properties. In cancer treatment, fusion proteins are used to deliver toxins directly to tumor cells, sparing healthy tissues and minimizing side effects. In autoimmune diseases, they act as immunomodulators by blocking pro-inflammatory cytokines or modulating immune responses to reduce symptoms. Fusion proteins are also utilized in enzyme replacement therapies (ERTs) for rare genetic disorders, where they replace deficient enzymes in patients. With their versatility, specificity, and potential to enhance drug delivery, fusion proteins have become a vital component in modern biopharmaceuticals, supporting precision medicine and innovative treatment approaches.
How Do Fusion Proteins Improve Therapeutic Efficacy & Drug Delivery?
Fusion proteins improve therapeutic efficacy by combining multiple biological functions into a single molecule, enabling targeted action and minimizing off-target effects. For example, in cancer immunotherapy, fusion proteins can be designed to link tumor-specific antigens with immune-activating domains, facilitating the targeted activation of immune cells against cancer cells. This targeted approach not only enhances the effectiveness of the treatment but also reduces the risk of adverse immune reactions. Similarly, fusion proteins that act as receptor antagonists or cytokine inhibitors can selectively block inflammatory pathways in autoimmune diseases, leading to better disease management with fewer side effects compared to traditional therapies.In drug delivery, fusion proteins offer improved pharmacokinetics, including extended half-life and enhanced bioavailability. By attaching a functional domain such as the Fc region of antibodies or albumin, fusion proteins achieve longer circulation time in the bloodstream, reducing the frequency of dosing and improving patient compliance. In enzyme replacement therapies (ERTs), fusion proteins are designed to enhance the delivery and stability of therapeutic enzymes, allowing for more efficient treatment of genetic disorders like Gaucher disease, Fabry disease, and mucopolysaccharidoses (MPS). Additionally, fusion proteins used as drug conjugates help in targeting specific cells or tissues, improving drug delivery and maximizing therapeutic impact while minimizing systemic toxicity. By offering targeted, longer-lasting, and efficient treatment options, fusion proteins enhance the overall effectiveness of biopharmaceuticals in addressing complex and chronic diseases.
How Are Technological Advancements Shaping the Development of Fusion Proteins?
Technological advancements have significantly improved the design, production, and clinical performance of fusion proteins, making them more effective for diverse therapeutic applications. One of the key innovations in this field is protein engineering, which enables the creation of highly specific and stable fusion proteins with optimized properties. Advances in molecular cloning, CRISPR-based gene editing, and synthetic biology have facilitated the precise design of fusion proteins, allowing for the development of novel constructs with enhanced potency, reduced immunogenicity, and improved stability. These technologies support the creation of next-generation fusion proteins, such as bispecific fusion proteins that can simultaneously bind two different targets, enhancing their therapeutic action in complex diseases like cancer and autoimmune disorders.Another major advancement is the development of novel expression systems and cell lines, such as mammalian, yeast, and insect cells, which improve the yield, purity, and scalability of fusion protein production. The use of glycoengineering has further enhanced the stability and bioactivity of fusion proteins by optimizing their glycosylation patterns, which is crucial for reducing immunogenicity and improving clinical outcomes. Additionally, innovations in linker technology have improved the functional connectivity between fused domains, ensuring that the combined protein retains the desired activity and targeting ability without compromising individual component functions.
The integration of computational biology and artificial intelligence (AI) has also accelerated the design and optimization of fusion proteins. AI-driven algorithms are used to predict the structural properties, folding patterns, and potential interactions of fusion proteins, enabling the rapid identification of candidates with high therapeutic potential. These computational tools aid in de-risking the drug development process and optimizing the performance of fusion proteins in preclinical and clinical trials. The development of advanced formulation technologies, such as sustained-release systems and nanocarriers, has further improved the delivery and efficacy of fusion proteins, making them more adaptable for different routes of administration, including intravenous, subcutaneous, and intramuscular injections. These technological advancements have not only enhanced the efficacy, safety, and versatility of fusion proteins but have also expanded their applications in oncology, immunology, rare diseases, and beyond.
What Factors Are Driving Growth in the Fusion Proteins Market?
The growth in the fusion proteins market is driven by several factors, including the rising prevalence of chronic diseases, increasing demand for targeted therapies, advancements in biotechnology, and expanding applications in drug discovery and development. The growing incidence of cancer, autoimmune disorders, and genetic diseases has created a strong demand for innovative treatments that offer better efficacy and fewer side effects. Fusion proteins, with their ability to target specific cells, modulate immune responses, and deliver therapeutic payloads directly to diseased tissues, meet the requirements of precision medicine and personalized treatment approaches.The increasing focus on biologics and protein-based therapies in the pharmaceutical industry has further fueled the adoption of fusion proteins, as they offer enhanced stability, longer half-life, and improved pharmacokinetics compared to small-molecule drugs. Regulatory approvals of successful fusion protein therapies, such as etanercept (Enbrel) for rheumatoid arthritis and aflibercept (Eylea) for age-related macular degeneration, have demonstrated the clinical and commercial viability of these biopharmaceuticals, encouraging investment and development in the sector. The expanding application of fusion proteins in drug conjugates, immuno-oncology, and gene therapy has also contributed to market growth, as these proteins provide novel mechanisms for delivering therapeutic effects with precision and efficiency.
Technological advancements, such as protein engineering, glycoengineering, and AI-driven drug design, have increased the speed and success rate of fusion protein development, driving wider adoption across therapeutic areas. Emerging markets, particularly in Asia-Pacific and Latin America, are witnessing increased investment in biotechnology infrastructure, creating new opportunities for fusion protein development and commercialization. Strategic collaborations between biotech firms, research institutes, and pharmaceutical companies have also accelerated the pipeline of fusion protein therapeutics, supporting rapid innovation and market expansion. With ongoing innovations, expanding clinical applications, and growing demand for targeted, effective therapies, the fusion proteins market is poised for sustained growth, driven by advancements in biotechnology, evolving healthcare needs, and global efforts toward personalized medicine.
Report Scope
The report analyzes the Fusion Proteins market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Application (Metabolic Disorders, Immunological Disorders, Hematological Disorders, Cancer, Hormonal Disorders, Genetic Disorders, Other Applications).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Metabolic Disorders Application segment, which is expected to reach US$12.9 Billion by 2030 with a CAGR of a 5.5%. The Immunological Disorders Application segment is also set to grow at 5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $8.9 Billion in 2024, and China, forecasted to grow at an impressive 4.5% CAGR to reach $6.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Fusion Proteins Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Fusion Proteins Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Fusion Proteins Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abnova, Absolute Antibody, Amgen Science, Astellas Pharma, Bristol-Myers Squibb and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Fusion Proteins market report include:
- Abnova
- Absolute Antibody
- Amgen Science
- Astellas Pharma
- Bristol-Myers Squibb
- Chimerigen
- Genzyme
- Ligand Pharmaceuticals
- NOVUS
- Peprotech
- ProSpec
- Regeneron
- Roche
- Viventia
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abnova
- Absolute Antibody
- Amgen Science
- Astellas Pharma
- Bristol-Myers Squibb
- Chimerigen
- Genzyme
- Ligand Pharmaceuticals
- NOVUS
- Peprotech
- ProSpec
- Regeneron
- Roche
- Viventia
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 131 |
| Published | January 2026 |
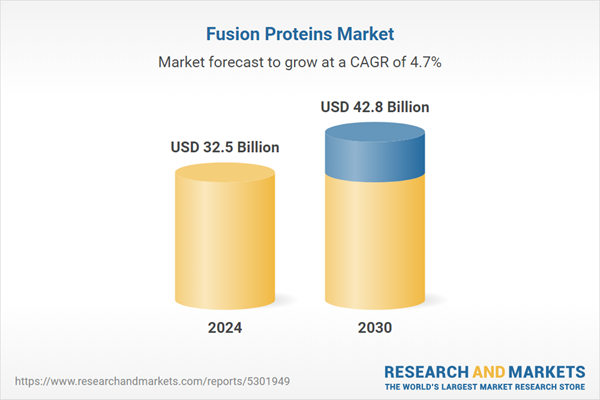
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 32.5 Billion |
| Forecasted Market Value ( USD | $ 42.8 Billion |
| Compound Annual Growth Rate | 4.7% |
| Regions Covered | Global |