Global Hernia Mesh Devices Market - Key Trends and Drivers Summarized
Why Are Hernia Mesh Devices Integral to Surgical Repairs?
Hernia mesh devices have become a standard solution for repairing hernias, a condition where internal organs or tissues push through weakened muscles. These devices, typically made from synthetic or biological materials, reinforce the damaged tissue and provide long-term support to prevent recurrence. Hernia mesh devices are widely used in both open and laparoscopic surgeries and are considered the most effective method for treating inguinal, ventral, and umbilical hernias. The growing number of hernia surgeries, driven by factors such as an aging population and increased rates of obesity, has expanded the demand for these devices. Mesh devices significantly reduce recovery time, improve patient outcomes, and lower the risk of hernia recurrence, making them indispensable in hernia repair procedures.How Are Technological Innovations Enhancing Hernia Mesh Devices?
The hernia mesh devices market has seen significant advancements in design and materials, leading to better patient outcomes. Modern meshes are now lighter, more flexible, and biocompatible, reducing the risk of complications such as infections and mesh migration. Advances in absorbable mesh technology have also contributed to improved healing, as these meshes naturally degrade over time, reducing the risk of long-term complications. Additionally, the development of 3D meshes tailored to fit specific anatomical structures has enhanced the precision and effectiveness of hernia repairs. Laparoscopic techniques that use mesh devices have also gained popularity due to their minimally invasive nature, which results in shorter recovery times and less post-surgical pain.How Are Market Segments Shaping the Growth of the Hernia Mesh Devices Market?
Product types include synthetic mesh, biological mesh, and composite mesh, with synthetic meshes holding the largest share due to their durability and cost-effectiveness. Biological meshes, made from human or animal tissue, are gaining traction for use in patients with higher infection risks. Surgery types are divided into open surgery and laparoscopic surgery, with laparoscopic procedures witnessing significant growth due to their minimally invasive approach and faster recovery times. In terms of material, non-absorbable meshes are more commonly used for permanent repairs, while absorbable meshes are preferred for temporary reinforcement.What Factors Are Driving the Growth in the Hernia Mesh Devices Market?
The growth in the hernia mesh devices market is driven by several factors, including the increasing prevalence of hernias, advancements in mesh technology, and the rise in minimally invasive surgeries. An aging population and the global rise in obesity rates have led to an increase in hernia cases, driving demand for effective treatment options. Technological advancements in mesh materials, including lightweight and biocompatible designs, have improved patient outcomes and reduced complications, further supporting market growth. The growing preference for laparoscopic surgeries, which use mesh devices for faster recovery and less postoperative pain, is also boosting demand. Additionally, the rise in healthcare expenditure and the expansion of healthcare infrastructure in emerging markets are contributing to the overall growth of the hernia mesh devices market.Report Scope
The report analyzes the Hernia Mesh Devices market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Hernia Type (Inguinal, Incisional, Other Hernia Types); Material (Synthetic, Biological).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Inguinal Hernia segment, which is expected to reach US$4.9 Billion by 2030 with a CAGR of a 3.9%. The Incisional Hernia segment is also set to grow at 3.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.3 Billion in 2024, and China, forecasted to grow at an impressive 5.6% CAGR to reach $1.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Hernia Mesh Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Hernia Mesh Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Hernia Mesh Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aspide Medical, Atrium, B. Braun Melsungen AG., Baxter International, Inc., C. R. Bard, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Hernia Mesh Devices market report include:
- Aspide Medical
- Atrium
- B. Braun Melsungen AG.
- Baxter International, Inc.
- C. R. Bard, Inc.
- Cook Medical
- Cousin Biotech
- Covidien (Part of Medtronic)
- Dipromed
- Ethicon, Inc.
- Feg Textiltechnik MBH
- Herniamesh S.r.l
- LifeCell Corporation
- Medtronic plc
- W. L. Gore & Associates
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aspide Medical
- Atrium
- B. Braun Melsungen AG.
- Baxter International, Inc.
- C. R. Bard, Inc.
- Cook Medical
- Cousin Biotech
- Covidien (Part of Medtronic)
- Dipromed
- Ethicon, Inc.
- Feg Textiltechnik MBH
- Herniamesh S.r.l
- LifeCell Corporation
- Medtronic plc
- W. L. Gore & Associates
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 270 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
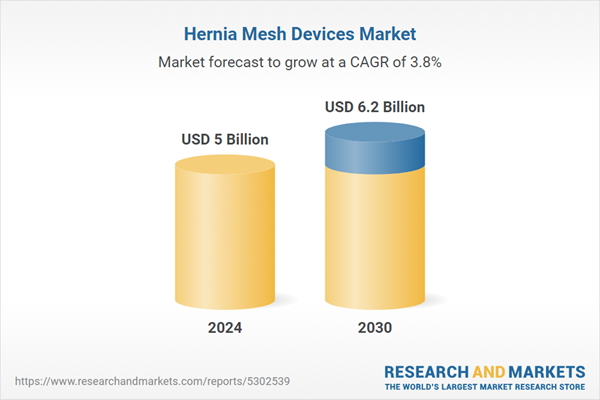
| Estimated Market Value ( USD | $ 5 Billion |
| Forecasted Market Value ( USD | $ 6.2 Billion |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | Global |