Global Hunter Syndrome Treatment Market - Key Trends and Drivers Summarized
What Is Hunter Syndrome and Why Is Its Treatment Complex?
Hunter Syndrome, or mucopolysaccharidosis type II (MPS II), is a rare genetic disorder caused by a deficiency of the enzyme iduronate-2-sulfatase, leading to the buildup of harmful molecules in the body. This condition primarily affects boys and results in a range of symptoms, including developmental delays, organ enlargement, skeletal abnormalities, and respiratory issues. The complexity of the disease lies in its progressive nature and the fact that symptoms vary widely from patient to patient, making treatment challenging. There is no cure for Hunter Syndrome, and the primary focus of current treatment is to manage symptoms and slow the progression of the disease through therapies like enzyme replacement and supportive care.How Are Treatment Options for Hunter Syndrome Evolving?
In recent years, the treatment landscape for Hunter Syndrome has expanded, particularly with the development of enzyme replacement therapy (ERT). ERT involves the intravenous administration of synthetic enzymes to compensate for the body's inability to produce them naturally, helping to reduce the buildup of glycosaminoglycans in tissues. However, this treatment does not cross the blood-brain barrier, limiting its effectiveness for patients with neurological symptoms. To address this, research is ongoing into gene therapy, which aims to deliver functional genes to produce the missing enzyme and provide a more comprehensive treatment option. Advances in pharmacological chaperones and substrate reduction therapies are also being explored to enhance the efficacy of current treatments and potentially slow disease progression more effectively.How Do Market Segments Influence the Hunter Syndrome Treatment Landscape?
Therapy types include enzyme replacement therapy, gene therapy, and substrate reduction therapy. Enzyme replacement therapy currently dominates the market due to its ability to address the primary cause of the disease, though its limitations are driving demand for more advanced therapies such as gene therapy. End-users include hospitals, specialty clinics, and research institutes, with hospitals accounting for the largest market share due to the need for specialized care and the complex nature of the treatments. The increasing focus on rare disease research and orphan drug development is also shaping the market, with pharmaceutical companies investing in innovative treatment solutions.What Factors Are Driving the Growth in the Hunter Syndrome Treatment Market?
The growth in the Hunter Syndrome treatment market is driven by several factors, including increasing awareness of rare diseases, advancements in therapeutic options such as gene therapy, and growing investments in orphan drug research. As early diagnosis of Hunter Syndrome improves, there is a greater demand for effective treatments to manage the disease and improve patient quality of life. The development of gene therapy, which offers the potential to address both the somatic and neurological symptoms of the disease, is a significant factor driving market growth. Furthermore, governmental incentives and funding for rare disease research are encouraging pharmaceutical companies to develop new treatments, further fueling the market's expansion.Report Scope
The report analyzes the Hunter Syndrome Treatment market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Treatment Type (Enzyme Replacement Therapy (ERT), Hematopoietic Stem Cell Transplant (HSCT), Other Treatment Types); End-Use (Hospitals, Clinics, Other End-Uses).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Enzyme Replacement Therapy (ERT) segment, which is expected to reach US$1.4 Billion by 2030 with a CAGR of a 6%. The Hematopoietic Stem Cell Transplant (HSCT) segment is also set to grow at 4.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $316.8 Million in 2024, and China, forecasted to grow at an impressive 9% CAGR to reach $399.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Hunter Syndrome Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Hunter Syndrome Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Hunter Syndrome Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ArmaGen, Inc., Bioasis Technologies, Inc., BioMarin Pharmaceutical, Inc, Canbridge Life Sciences Ltd., Denali Therapeutics, Inc and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Hunter Syndrome Treatment market report include:
- ArmaGen, Inc.
- Bioasis Technologies, Inc.
- BioMarin Pharmaceutical, Inc
- Canbridge Life Sciences Ltd.
- Denali Therapeutics, Inc
- GC Pharma
- Genzyme
- Green Cross Corp.
- Inventiva S.A.
- JCR Pharmaceuticals Co., Ltd.
- RegenxBio, Inc.
- Sangamo Therapeutics, Inc.
- Shrine Plc
- Takeda Pharmaceutical Company
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ArmaGen, Inc.
- Bioasis Technologies, Inc.
- BioMarin Pharmaceutical, Inc
- Canbridge Life Sciences Ltd.
- Denali Therapeutics, Inc
- GC Pharma
- Genzyme
- Green Cross Corp.
- Inventiva S.A.
- JCR Pharmaceuticals Co., Ltd.
- RegenxBio, Inc.
- Sangamo Therapeutics, Inc.
- Shrine Plc
- Takeda Pharmaceutical Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 280 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
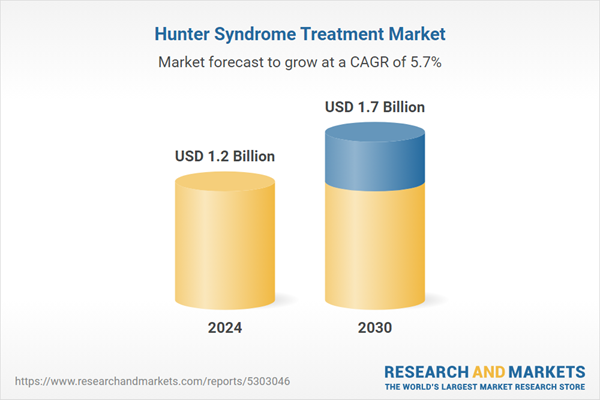
| Estimated Market Value ( USD | $ 1.2 Billion |
| Forecasted Market Value ( USD | $ 1.7 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |