Global Paroxysmal Nocturnal Hemoglobinuria (PNH) Treatment Market - Key Trends & Drivers Summarized
Why Is Effective Treatment for Paroxysmal Nocturnal Hemoglobinuria (PNH) Essential?
Effective treatment for Paroxysmal Nocturnal Hemoglobinuria (PNH) is essential due to the severe and life-threatening complications associated with this rare blood disorder. PNH causes red blood cells to break apart prematurely, leading to hemolytic anemia, thrombosis, and, in severe cases, organ damage and death. Since the disease involves immune-mediated destruction of red blood cells, it places patients at constant risk for debilitating symptoms such as fatigue, abdominal pain, difficulty breathing, and blood clots. Without treatment, these complications can severely impact patients' quality of life and may result in life-threatening events like strokes and organ failure. Treatments for PNH focus on preventing hemolysis, reducing thrombotic events, and managing symptoms to improve patient outcomes.PNH treatment typically involves therapies that target the immune system, specifically complement inhibitors, which prevent the immune system from attacking red blood cells. The introduction of complement inhibitors like eculizumab (Soliris) and ravulizumab (Ultomiris) has transformed PNH management by reducing the rate of hemolysis and decreasing the risk of thrombosis, providing patients with longer life expectancy and better quality of life. These treatments have become essential for managing the disease and preventing the serious complications associated with uncontrolled hemolysis. Given the high risk of thrombotic events in PNH, especially in veins, complement inhibitors are critical in reducing these complications and allowing patients to lead more stable lives.
In addition to complement inhibitors, supportive care plays a significant role in managing PNH, including blood transfusions to manage anemia and medications to prevent clot formation. Blood transfusions can help maintain hemoglobin levels, addressing anemia-related symptoms like fatigue and weakness, while anticoagulants can reduce the risk of blood clots. Advances in PNH treatment focus on reducing the need for supportive care and improving the efficacy of complement inhibitors. As the disease's complexity and risks require a multifaceted approach, effective treatments are vital for stabilizing patients, preventing complications, and ultimately enhancing the quality of life for those affected by PNH.
How Are Technological and Pharmacological Advancements Transforming PNH Treatment?
Technological and pharmacological advancements are transforming PNH treatment by offering more effective therapies with longer-lasting effects, improved safety, and enhanced patient convenience. One of the most significant innovations in PNH treatment is the development of long-acting complement inhibitors, such as ravulizumab, which allow for less frequent dosing than earlier treatments like eculizumab. Ravulizumab extends the dosing interval from every two weeks to every eight weeks, providing patients with greater convenience and improving adherence to treatment. This extended dosing schedule is especially beneficial for patients who find frequent hospital visits challenging and time-consuming. By reducing the burden of frequent infusions, long-acting complement inhibitors support more manageable treatment routines and provide a more patient-friendly experience.New complement inhibitors targeting alternative pathways, such as C3 inhibitors and alternative pathway inhibitors, are emerging as promising options for patients who are resistant to existing treatments. Zilucoplan and pegcetacoplan, for example, are designed to block the complement pathway earlier in the cascade, providing broader inhibition that may benefit patients with partial or inadequate responses to traditional C5 inhibitors. These alternative pathway inhibitors aim to prevent hemolysis more effectively and reduce the need for blood transfusions, offering hope for patients who do not achieve full remission with current therapies. As these new inhibitors reach advanced clinical trials, they hold potential to expand PNH treatment options, providing a solution for those who require more comprehensive complement inhibition.
Gene therapy and CRISPR-based research are also advancing as potential long-term solutions for PNH, with the goal of correcting the genetic defect that causes the disease. Although still in early stages of research, gene therapy aims to provide a one-time treatment that can potentially cure or significantly reduce the need for ongoing medication. By directly targeting the genetic mutation that leads to PNH, gene therapy could offer a permanent solution for patients. While these technologies are still under development, their potential for long-lasting efficacy makes them an exciting area of research. As gene therapy advances, it may revolutionize PNH treatment, offering patients hope for a future without lifelong reliance on medication.
What Are the Benefits of PNH Treatments for Patients and Healthcare Systems?
PNH treatments provide significant benefits for patients by managing symptoms, reducing the risk of life-threatening complications, and improving quality of life, while healthcare systems benefit from reduced hospitalization and improved patient outcomes. For patients, complement inhibitors like eculizumab and ravulizumab have transformed PNH management by reducing hemolysis, which alleviates symptoms such as fatigue, weakness, and difficulty breathing. By reducing hemolysis, these treatments help patients maintain healthier hemoglobin levels, enhancing their ability to participate in daily activities. For patients with PNH, effective symptom management is critical for maintaining independence and reducing the impact of the disease on their daily lives.Complement inhibitors also play a crucial role in reducing the risk of thrombosis, which is one of the leading causes of mortality in PNH patients. By preventing clot formation, these treatments decrease the likelihood of severe complications such as strokes, pulmonary embolisms, and organ damage. This reduction in thrombotic events is particularly beneficial for patients' long-term survival, as untreated thrombosis can lead to fatal outcomes. Effective treatment with complement inhibitors thus reduces the need for emergency interventions, improving the stability of patients' health and providing a better long-term prognosis.
For healthcare systems, PNH treatments reduce the need for frequent hospitalizations and emergency care, lowering overall healthcare costs associated with managing complications. As treatments like ravulizumab allow for less frequent dosing, they decrease the frequency of hospital visits and infusion sessions, freeing up healthcare resources and improving cost efficiency. By providing effective, long-acting solutions, healthcare systems can focus on preventive care rather than acute interventions, which supports better patient outcomes. Furthermore, as new treatments improve the effectiveness of PNH management, patients are less reliant on supportive care like blood transfusions and anticoagulants, which further reduces healthcare expenses and resource allocation.
Additionally, the development of oral complement inhibitors in clinical trials could offer even greater convenience and access to treatment for patients, especially in regions where access to infusion centers is limited. Oral therapies would allow patients to manage their condition from home, eliminating the need for frequent travel to healthcare facilities. This accessibility can greatly benefit rural or underserved populations, providing them with the necessary treatment without the logistical and financial burden of travel. For healthcare systems, oral therapies would reduce the demand on infusion centers and hospital resources, further supporting efficient care delivery and improving accessibility for all PNH patients.
What Is Fueling the Growth in the PNH Treatment Market?
The growth in the PNH treatment market is driven by increased awareness of rare diseases, advancements in targeted therapies, expanded patient access to new treatments, and the emergence of gene therapy research. With greater awareness and understanding of PNH among healthcare providers and patients, diagnosis rates have improved, leading to earlier and more accurate treatment initiation. As patients are diagnosed sooner, they can begin receiving life-saving treatments that reduce complications and improve long-term outcomes. Awareness efforts, often driven by patient advocacy groups, have also encouraged more investment in PNH research, leading to the development of advanced therapies and expanded treatment options. As awareness and diagnosis improve, the demand for effective PNH treatments continues to grow.Advancements in targeted therapies, particularly in complement inhibitors, are a major growth driver, as these treatments offer effective control of hemolysis and reduce the risk of thrombotic events. The success of C5 inhibitors like eculizumab and ravulizumab has paved the way for the development of next-generation therapies, including C3 inhibitors and other complement pathway inhibitors. These new therapies are expanding treatment options and addressing unmet needs for patients with incomplete responses to existing treatments. The market's growth is further supported by increasing interest in rare disease research, which has encouraged pharmaceutical companies to invest in innovative treatments for PNH. As companies pursue novel therapies and expand the range of complement inhibitors, the PNH treatment market is set to grow.
Expanded patient access to PNH treatments, facilitated by regulatory approvals and broader insurance coverage, is also driving market growth. Regulatory agencies such as the FDA and EMA have granted orphan drug status to many PNH treatments, streamlining approval processes and increasing availability in different regions. Insurance coverage for high-cost therapies like complement inhibitors has also improved, making these treatments more accessible to patients who previously may not have been able to afford them. Additionally, the introduction of long-acting therapies, which require less frequent dosing, has made treatment more convenient and accessible for patients, particularly those in remote or underserved areas. As access to PNH treatments expands globally, the market is expected to grow, reaching more patients and improving outcomes across diverse regions.
The emergence of gene therapy research holds promise for transforming the PNH treatment market in the long term. By addressing the underlying genetic mutation that causes PNH, gene therapy has the potential to offer a one-time, curative treatment that eliminates the need for lifelong medication. Although still in early stages, gene therapy research has garnered significant interest due to its potential to provide long-lasting relief from PNH symptoms and complications. Investment in gene therapy and CRISPR-based solutions has increased as companies explore their application in PNH treatment. Should these therapies prove successful, they would revolutionize the PNH market, offering a permanent solution for patients and reducing reliance on costly, ongoing treatments. Together, these factors - greater awareness, targeted therapeutic advancements, increased patient access, and gene therapy research - are driving robust growth in the PNH treatment market, establishing it as a key area in rare disease management and innovative healthcare.
Report Scope
The report analyzes the Paroxysmal Nocturnal Hemoglobinuria (PNH) Treatment market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Treatment (Medication, Other Treatments).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Medication segment, which is expected to reach US$5.5 Billion by 2030 with a CAGR of a 9.1%. The Other Treatments segment is also set to grow at 14.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.2 Billion in 2024, and China, forecasted to grow at an impressive 10.2% CAGR to reach $1.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Paroxysmal Nocturnal Hemoglobinuria (PNH) Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Paroxysmal Nocturnal Hemoglobinuria (PNH) Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Paroxysmal Nocturnal Hemoglobinuria (PNH) Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Akari Therapeutics Plc, Alexion Pharmaceuticals, Inc., Apellis Pharmaceuticals, AstraZeneca Plc, CANbridge Pharmaceuticals and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Paroxysmal Nocturnal Hemoglobinuria (PNH) Treatment market report include:
- Akari Therapeutics Plc
- Alexion Pharmaceuticals, Inc.
- Apellis Pharmaceuticals
- AstraZeneca Plc
- CANbridge Pharmaceuticals
- Chugai Pharmaceutical Co., Ltd.
- F. Hoffmann-La Roche AG
- Genentech, Inc.
- Kira Pharmaceuticals
- Novartis AG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Akari Therapeutics Plc
- Alexion Pharmaceuticals, Inc.
- Apellis Pharmaceuticals
- AstraZeneca Plc
- CANbridge Pharmaceuticals
- Chugai Pharmaceutical Co., Ltd.
- F. Hoffmann-La Roche AG
- Genentech, Inc.
- Kira Pharmaceuticals
- Novartis AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 126 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
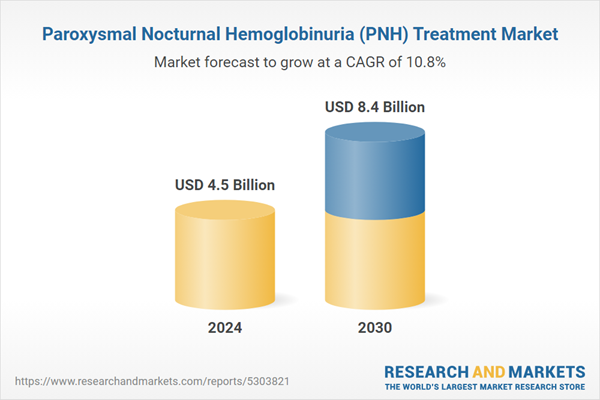
| Estimated Market Value ( USD | $ 4.5 Billion |
| Forecasted Market Value ( USD | $ 8.4 Billion |
| Compound Annual Growth Rate | 10.8% |
| Regions Covered | Global |