Global Preclinical Contract Research Organizations Market - Key Trends & Drivers Summarized
How Are Preclinical Contract Research Organizations (CROs) Shaping The Drug Development Process?
Preclinical Contract Research Organizations (CROs) play a critical role in the drug development pipeline, providing pharmaceutical and biotechnology companies with essential services to support early-stage research. These organizations specialize in conducting preclinical studies that assess the safety, efficacy, and pharmacokinetics of new drug candidates before they proceed to clinical trials. The services offered by preclinical CROs include in vitro and in vivo studies, toxicology testing, pharmacokinetics (PK) and pharmacodynamics (PD) evaluations, bioanalytical services, and regulatory consulting. By outsourcing these complex and resource-intensive activities to specialized CROs, drug developers can streamline their research processes, reduce costs, and focus on core competencies like drug discovery and strategic planning.The importance of preclinical CROs has grown in tandem with the increasing complexity of drug development. Today’s biopharmaceutical landscape is characterized by the development of more specialized and advanced therapies, such as biologics, gene therapies, and personalized medicine. These therapies require highly specialized preclinical testing protocols to evaluate their safety and therapeutic potential. CROs offer the infrastructure, expertise, and regulatory knowledge necessary to conduct these tests in accordance with Good Laboratory Practice (GLP) standards. As the drug development process becomes more global and regulatory requirements become more stringent, preclinical CROs serve as invaluable partners to companies seeking to navigate these challenges and bring new therapies to market efficiently.
What Technological Innovations Are Driving The Growth Of Preclinical CROs?
Technological advancements are playing a pivotal role in enhancing the capabilities and efficiency of preclinical Contract Research Organizations. One of the most significant innovations impacting this sector is the increased adoption of advanced in silico modeling and simulation techniques. These computational tools allow CROs to predict the biological behavior of drug candidates before initiating costly and time-consuming in vivo studies. By modeling pharmacokinetics, toxicity, and efficacy, these technologies help reduce the number of compounds that progress to animal testing, thereby saving both time and resources. Artificial intelligence (AI) and machine learning are also being integrated into preclinical research to analyze vast datasets, identify potential drug candidates, and optimize study designs.Another major technological advancement is the growing use of high-throughput screening (HTS) and automated systems in preclinical testing. Automated platforms allow CROs to rapidly screen thousands of compounds for their biological activity, providing quicker results and reducing human error. This automation is particularly valuable for large-scale pharmacokinetics and toxicology studies, where speed and accuracy are critical. Additionally, advancements in imaging technologies, such as positron emission tomography (PET) and magnetic resonance imaging (MRI), are enabling more detailed and real-time monitoring of drug effects in animal models, enhancing the accuracy of preclinical studies.
The emergence of 3D cell culture and organ-on-a-chip technologies is also transforming the preclinical landscape. These technologies provide more physiologically relevant models that better mimic human biology compared to traditional 2D cell cultures. By using 3D cell cultures and microfluidic devices that simulate the behavior of human tissues and organs, CROs can conduct more accurate and predictive studies on drug efficacy and toxicity. This not only improves the reliability of preclinical results but also supports the development of more complex therapies like biologics and personalized medicines.
How Are Market Dynamics And Outsourcing Trends Shaping The Role Of Preclinical CROs?
The growing trend of outsourcing preclinical research to CROs is reshaping the global pharmaceutical and biotechnology industries. As drug development costs continue to rise and timelines become more stringent, companies are increasingly looking to preclinical CROs to provide specialized expertise and cost-effective solutions. Outsourcing allows pharmaceutical companies to mitigate the high financial risk associated with preclinical studies, as CROs provide the necessary infrastructure, technology, and regulatory expertise to execute these studies efficiently. This shift toward outsourcing has led to a growing reliance on CROs for comprehensive drug development services, from discovery through to early clinical trials.One of the key factors driving this trend is the increasing specialization of drug development, particularly with the rise of personalized medicine, gene therapies, and cell-based treatments. These therapies require preclinical testing protocols that go beyond traditional small-molecule drugs, necessitating the use of CROs with specialized capabilities in areas like immunology, genetic engineering, and bioinformatics. Additionally, as global pharmaceutical companies expand their operations across different regions, they often turn to CROs with a strong presence in specific markets to help navigate regional regulatory requirements and conduct studies in diverse patient populations. The ability of preclinical CROs to manage these complexities allows drug developers to focus on their core research and development efforts while maintaining compliance with regulatory standards.
Moreover, the consolidation of the pharmaceutical industry, with increasing mergers and acquisitions, has also contributed to the rise of preclinical CROs. Smaller biotechnology companies, which may lack the financial and operational resources to conduct in-house preclinical studies, often partner with CROs to advance their drug candidates through the early stages of development. Large pharmaceutical companies also benefit from CRO partnerships by optimizing their resources and focusing on late-stage clinical trials, where the financial stakes are higher. The flexibility and scalability that CROs offer make them attractive partners in a fast-paced, competitive market.
What Factors Are Driving Growth In The Preclinical CRO Market?
The growth in thePreclinical Contract Research Organization (CRO) market is driven by several factors, including technological advancements, rising demand for outsourced services, and the increasing complexity of drug development. One of the primary growth drivers is the growing prevalence of complex and advanced therapies such as biologics, gene therapies, and personalized medicine. These therapies require specialized preclinical testing capabilities, including advanced toxicology, immunogenicity testing, and genetic profiling, which CROs are uniquely positioned to offer. As these therapies become more prevalent, the need for CROs with expertise in these areas continues to grow.
Another key driver of market growth is the rising cost of in-house preclinical testing, which has led pharmaceutical and biotechnology companies to outsource these functions to CROs. Preclinical studies are often resource-intensive, requiring sophisticated laboratory infrastructure, skilled personnel, and strict adherence to regulatory standards. By outsourcing to CROs, companies can reduce capital expenditures, shorten development timelines, and allocate their internal resources to strategic areas of drug discovery and clinical trials. This trend is particularly pronounced among smaller biotech firms and startups, which rely heavily on outsourcing to advance their drug candidates through the preclinical pipeline.
The global expansion of the pharmaceutical industry and the increasing complexity of regulatory requirements are also contributing to the growth of the preclinical CRO market. As pharmaceutical companies seek to enter new markets, they must comply with varying regional regulatory standards. CROs with a strong understanding of local regulations and experience in navigating the complexities of international drug development are in high demand. Additionally, the push for faster drug approvals and the rise of orphan drug designations are driving the need for more efficient preclinical testing, further fueling demand for CRO services.
Lastly, the growing emphasis on reducing animal testing and improving the predictive accuracy of preclinical models is encouraging the adoption of advanced technologies like in silico modeling, organ-on-a-chip systems, and 3D cell cultures. These innovations are reshaping the preclinical research landscape and creating new opportunities for CROs to differentiate their offerings. As these technologies gain wider acceptance and regulatory support, they are expected to drive further growth in the preclinical CRO market, particularly among companies developing cutting-edge therapies that require sophisticated preclinical validation.
SCOPE OF STUDY:
The report analyzes the Preclinical Contract Research Organizations market in terms of units by the following Segments, and Geographic Regions/Countries:- Segments: Service (Toxicology Testing, Bioanalysis & DMPK Studies, Compound Management, Chemistry, Safety Pharmacology, Other Services); End-Use (Biopharma Companies, Government & Academic Institutes, Medical Device Companies)
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Toxicology Testing segment, which is expected to reach US$3.2 Billion by 2030 with a CAGR of a 7.4%. The Bioanalysis & DMPK Studies segment is also set to grow at 7.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.1 Billion in 2024, and China, forecasted to grow at an impressive 6.6% CAGR to reach $1.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Preclinical Contract Research Organizations Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Preclinical Contract Research Organizations Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Preclinical Contract Research Organizations Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Envigo Corporation, Eurofins Scientific, ICON Plc, Laboratory Corporation of America, Inc., Medpace, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 33 companies featured in this Preclinical Contract Research Organizations market report include:
- Envigo Corporation
- Eurofins Scientific
- ICON Plc
- Laboratory Corporation of America, Inc.
- Medpace, Inc.
- MPI research
- PARAXEL International Corporation
- Pharmaceutical Product Development (PPD), LLC
- PRA Health Sciences, Inc.
- Wuxi AppTec
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Envigo Corporation
- Eurofins Scientific
- ICON Plc
- Laboratory Corporation of America, Inc.
- Medpace, Inc.
- MPI research
- PARAXEL International Corporation
- Pharmaceutical Product Development (PPD), LLC
- PRA Health Sciences, Inc.
- Wuxi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 177 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
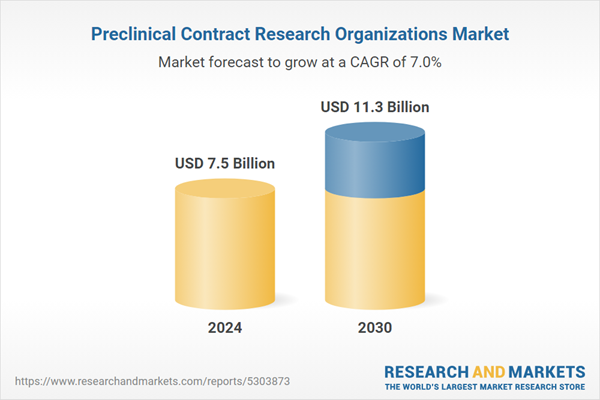
| Estimated Market Value ( USD | $ 7.5 Billion |
| Forecasted Market Value ( USD | $ 11.3 Billion |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |