Global Pharmaceutical Filtration Market - Key Trends & Drivers Summarized
How Has the Pharmaceutical Filtration Market Evolved?
Pharmaceutical filtration plays a crucial role in the production and purification of drug formulations, ensuring that pharmaceutical products are free from contaminants, particles, and microorganisms. Over the years, the pharmaceutical filtration market has evolved significantly, driven by advancements in drug manufacturing processes and stringent regulatory requirements. Initially, filtration techniques in the pharmaceutical industry were primarily focused on basic filtration needs, such as removing solid particles from liquids. However, as pharmaceutical manufacturing has become more complex, the scope of filtration has expanded to include microfiltration, nanofiltration, ultrafiltration, and other advanced processes designed to meet the stringent purity standards required in modern drug production.The filtration process is particularly critical in the production of sterile injectable drugs, biologics, vaccines, and other sensitive formulations, where even trace levels of impurities could compromise drug safety or efficacy. The growing focus on biologics and biosimilars, which are highly sensitive to contamination, has further emphasized the need for sophisticated filtration solutions. As a result, the market for pharmaceutical filtration has seen increased demand from biopharmaceutical companies, contract manufacturing organizations (CMOs), and research institutions seeking advanced, reliable, and scalable filtration systems to meet regulatory and quality control standards.
How Are Technological Advancements Shaping the Pharmaceutical Filtration Market?
Technological advancements are playing a transformative role in the pharmaceutical filtration market, improving filtration efficiency, enhancing scalability, and ensuring compliance with increasingly stringent regulatory requirements. One of the most significant innovations in this sector is the development of single-use filtration systems, which have gained widespread adoption in biopharmaceutical manufacturing. Single-use filtration eliminates the need for cleaning and validation between batches, reducing the risk of cross-contamination and streamlining the production process. These systems also offer flexibility in manufacturing, allowing companies to scale production up or down depending on demand without investing in costly infrastructure. Membrane filtration technologies, such as microfiltration, ultrafiltration, and nanofiltration, have also advanced significantly. These technologies allow for the precise removal of particles, bacteria, and other impurities from liquids and gases, making them essential in the production of sterile drugs and biopharmaceuticals. Advances in membrane materials, including ceramic and polymer membranes, have improved filtration performance, durability, and resistance to harsh chemicals or extreme conditions. Additionally, automated filtration systems integrated with real-time monitoring and data analytics capabilities are becoming more prevalent. These systems help manufacturers optimize filtration processes, ensuring that they meet quality standards and regulatory requirements while reducing operational costs. Another technological advancement impacting the market is continuous filtration, which allows for uninterrupted filtration processes. Continuous filtration is especially beneficial in large-scale drug manufacturing, where stopping and restarting the process could lead to inefficiencies and increased costs. This method is now being adopted by biopharmaceutical manufacturers looking to enhance their production efficiency and meet growing demand for biologics and vaccines.What Are the Emerging Trends in the Pharmaceutical Filtration Market?
Several emerging trends are reshaping the pharmaceutical filtration market as it responds to growing industry needs and regulatory pressures. One major trend is the increasing focus on biologics and biosimilars in drug development. As biologics are more complex and sensitive than traditional small-molecule drugs, they require highly sophisticated filtration techniques to ensure the removal of contaminants while preserving the integrity of the drug. The rising demand for biologics, including monoclonal antibodies, vaccines, and gene therapies, has significantly boosted the need for specialized filtration systems designed for these sensitive products. Another important trend is the growing use of disposable or single-use technologies (SUTs) in pharmaceutical manufacturing. Single-use filtration systems offer several advantages, including reduced downtime, lower risk of cross-contamination, and easier scalability, making them ideal for small batch production and clinical trial manufacturing. As more pharmaceutical companies adopt flexible manufacturing models, especially in the production of personalized medicines and cell therapies, single-use filtration technologies are expected to see continued growth. Sustainability is also becoming a key consideration in the pharmaceutical filtration market. Manufacturers are looking for ways to minimize waste, reduce energy consumption, and use environmentally friendly materials in their filtration processes. There is a growing interest in filtration systems that incorporate recyclable materials or have a smaller environmental footprint. This focus on sustainability is being driven by both regulatory pressures and consumer demand for more eco-friendly pharmaceutical production practices. The integration of digital technologies and process automation is another trend shaping the market. Advanced sensors, data analytics, and machine learning algorithms are being integrated into filtration systems to provide real-time monitoring and control of filtration processes. This not only improves the efficiency and consistency of the filtration process but also ensures that manufacturers can meet stringent regulatory requirements while reducing the risk of errors or contamination.What Is Driving the Growth of the Pharmaceutical Filtration Market?
The growth in the pharmaceutical filtration market is driven by several key factors, including the rising demand for biologics, increasing regulatory scrutiny, and technological advancements in filtration systems. One of the most important drivers is the expansion of biologics production, which has created a significant need for advanced filtration solutions. Biopharmaceuticals, which include monoclonal antibodies, vaccines, and cell and gene therapies, require highly stringent filtration processes to ensure product purity and safety. The increasing focus on biologics, combined with the growth of biosimilars, is expected to fuel demand for filtration systems that are designed specifically for these complex therapies. Another major factor is the stringent regulatory environment governing pharmaceutical manufacturing. Regulatory agencies such as the U.S. FDA, EMA, and other global authorities have imposed strict guidelines for drug production, particularly regarding sterility and contamination control. Pharmaceutical filtration systems play a critical role in helping manufacturers meet these regulatory requirements by ensuring that products are free from particulate matter, microorganisms, and pyrogens. As regulatory standards become more rigorous, pharmaceutical companies are investing heavily in advanced filtration technologies to ensure compliance and maintain product safety. The adoption of single-use technologies (SUTs) is also a key growth driver. Single-use filtration systems offer greater flexibility, efficiency, and cost-effectiveness, particularly in biopharmaceutical production. These systems reduce the need for cleaning and validation, thereby lowering operational costs and shortening production timelines. The growing trend toward personalized medicines, which often require small batch production, is further boosting the demand for single-use filtration systems. Additionally, technological innovations such as continuous filtration, automated systems, and advanced membrane materials are driving the market forward. These innovations enable pharmaceutical companies to improve the efficiency, scalability, and reliability of their filtration processes. The focus on sustainability in pharmaceutical manufacturing is another driver, as companies seek eco-friendly solutions that minimize waste and energy consumption. Combined, these factors are expected to contribute to sustained growth in the pharmaceutical filtration market as demand for high-quality, safe, and efficient drug production processes continues to rise.Report Scope
The report analyzes the Pharmaceutical Filtration market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Membrane Filters, Prefilters & Depth Media, Single-Use Systems, Cartridges & Capsules, Filter Holders, Other Products); Technique (Microfiltration, Ultrafiltration, Nanofiltration, Other Techniques); Application (Final Product Processing, Raw Material Filtration, Cell Separation, Water Purification, Air Purification).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Membrane Filters segment, which is expected to reach US$19.6 Billion by 2030 with a CAGR of a 21.2%. The Prefilters & Depth Media segment is also set to grow at 17.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.9 Billion in 2024, and China, forecasted to grow at an impressive 22.7% CAGR to reach $14.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pharmaceutical Filtration Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pharmaceutical Filtration Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pharmaceutical Filtration Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3M Healthcare Ltd., A One Engineering Works, Across International, Hawach Scientific Co., Ltd. (Filter Membrane), Hongtek Filtration Co., Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Pharmaceutical Filtration market report include:
- 3M Healthcare Ltd.
- A One Engineering Works
- Across International
- Hawach Scientific Co., Ltd. (Filter Membrane)
- Hongtek Filtration Co., Ltd.
- KEWLAB PTY LTD.
- Meissner Filtration Products
- Metrohm India Limited
- Micronics Engineered Filtration Group, Inc.
- Stevanato Group
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M Healthcare Ltd.
- A One Engineering Works
- Across International
- Hawach Scientific Co., Ltd. (Filter Membrane)
- Hongtek Filtration Co., Ltd.
- KEWLAB PTY LTD.
- Meissner Filtration Products
- Metrohm India Limited
- Micronics Engineered Filtration Group, Inc.
- Stevanato Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 393 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
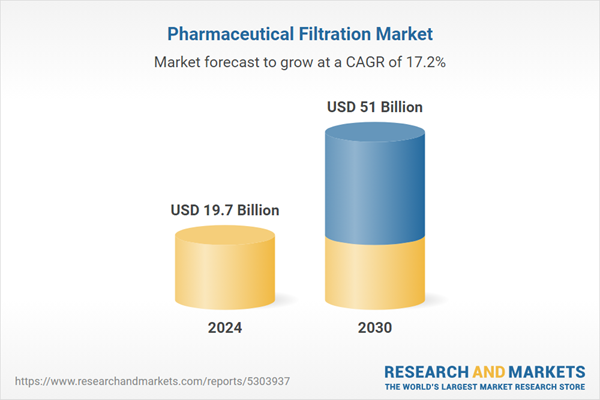
| Estimated Market Value ( USD | $ 19.7 Billion |
| Forecasted Market Value ( USD | $ 51 Billion |
| Compound Annual Growth Rate | 17.2% |
| Regions Covered | Global |