Global Pharmaceutical Analytical Testing Outsourcing Market - Key Trends & Drivers Summarized
How Has the Pharmaceutical Analytical Testing Outsourcing Industry Evolved Over Time?
The pharmaceutical analytical testing outsourcing industry has undergone a profound evolution over the past two decades, primarily driven by the increasing complexity of drug development, regulatory scrutiny, and the need for cost efficiencies. Traditionally, pharmaceutical companies conducted most of their analytical testing in-house, managing all stages of drug development from research to market entry. However, as drug pipelines have grown more complex and regulatory demands have increased, many companies have begun outsourcing these specialized testing functions to external partners. This shift allows pharmaceutical companies to focus on their core competencies, such as drug discovery and commercialization, while leveraging the expertise of contract research organizations (CROs) for analytical testing services.Today, outsourcing has become a vital component of pharmaceutical research and development (R&D). Pharmaceutical analytical testing encompasses a wide range of services including raw material testing, method development, stability testing, bioanalytical services, and product release testing. Outsourcing these services has allowed pharmaceutical companies to access state-of-the-art technology, advanced instrumentation, and regulatory expertise without the need for significant capital investment. As a result, the outsourcing market has grown exponentially, with CROs offering a comprehensive suite of services to meet the demands of both small biotech firms and large pharmaceutical companies alike.
How Are Technological Advancements Shaping the Pharmaceutical Analytical Testing Outsourcing Market?
Technological advancements are playing a critical role in shaping the pharmaceutical analytical testing outsourcing market by improving the accuracy, efficiency, and speed of testing processes. One of the most significant innovations driving this market is the adoption of advanced analytical instruments and high-throughput screening technologies. These instruments, such as liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) spectroscopy, offer higher precision and faster analysis times, enabling CROs to handle large volumes of samples quickly and accurately. These technologies are particularly important for the identification and quantification of complex molecules and impurities, making them indispensable in pharmaceutical testing. In addition to instrumentation, automation and robotics have revolutionized laboratory workflows, allowing for greater efficiency and reducing the chances of human error. Automated systems can manage large volumes of repetitive tasks such as sample preparation, data collection, and analysis, ensuring consistent and reproducible results. This has become particularly valuable in areas like high-throughput screening and stability testing, where thousands of samples may need to be processed simultaneously. Additionally, the integration of cloud computing and data analytics has enabled pharmaceutical companies and CROs to store, share, and analyze data more efficiently, streamlining collaboration across global teams and ensuring compliance with data integrity regulations.What Are the Key Market Trends Driving Outsourcing in Pharmaceutical Analytical Testing?
Several key market trends are driving the growth of outsourcing in pharmaceutical analytical testing. The increasing focus on biologics and biosimilars has significantly impacted the demand for advanced analytical testing services. Biologics, which are larger and more complex than traditional small-molecule drugs, require more rigorous and specialized testing protocols to ensure quality, efficacy, and safety. CROs with expertise in bioanalytical testing are becoming essential partners for pharmaceutical companies developing these therapies, especially as regulatory requirements for biologics become more stringent. Another major trend influencing the market is the rising regulatory scrutiny in drug development. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are enforcing stricter guidelines on drug safety, quality, and efficacy, prompting pharmaceutical companies to seek out CROs that can provide expertise in regulatory compliance and high-quality testing. This includes testing for impurities, stability, and degradation, as well as ensuring that manufacturing processes adhere to Good Manufacturing Practices (GMP). The increasing prevalence of specialty and personalized medicines is also driving the need for more specialized testing services. Personalized therapies, particularly those based on genetics or rare diseases, often require highly specific analytical methods, which many pharmaceutical companies may not have the in-house capability to perform. As a result, these companies are increasingly outsourcing their testing needs to CROs that specialize in niche areas of drug development. Additionally, the shift toward virtual pharma models, where companies focus exclusively on drug development and outsource all other functions, has further accelerated the demand for comprehensive analytical testing services.Key Factors Fueling the Growth of the Pharmaceutical Analytical Testing Outsourcing Market
The growth in the pharmaceutical analytical testing outsourcing market is driven by several factors, each contributing to the increasing reliance on external service providers. One of the key drivers is the rising complexity of drug molecules, particularly in the biologics and biosimilars sector. These drugs require more sophisticated analytical testing methods to ensure their safety, purity, and efficacy, which often necessitates the use of highly specialized equipment and expertise that pharmaceutical companies may lack internally. This has prompted the outsourcing of these tasks to CROs that possess the technological capabilities and regulatory know-how to handle complex biologics testing. Another critical factor fueling growth is the cost-saving potential that outsourcing offers pharmaceutical companies. By outsourcing analytical testing, companies can avoid significant capital investment in expensive equipment and infrastructure while also reducing the costs associated with staffing and maintaining in-house laboratories. This is especially appealing to smaller biotech firms or start-ups that may lack the resources to develop in-house testing capabilities but require access to high-quality analytical services to advance their drug candidates. Regulatory pressures are also driving the demand for outsourcing, as pharmaceutical companies face increasingly stringent requirements from regulatory agencies worldwide. CROs with expertise in regulatory compliance offer a distinct advantage by ensuring that testing processes meet global standards, mitigating the risk of delays or rejections during the approval process. Additionally, the growing trend toward globalization in drug development means that pharmaceutical companies are working across multiple geographic regions, each with its own regulatory requirements. This further increases the reliance on CROs that have a global footprint and the ability to navigate complex regulatory landscapes. Lastly, the rapid advancements in digital technologies and data analytics have made outsourcing an attractive option for pharmaceutical companies looking to optimize their R&D processes. CROs that offer advanced data analytics capabilities can provide deeper insights into testing results, accelerating decision-making and improving the overall efficiency of drug development.Report Scope
The report analyzes the Pharmaceutical Analytical Testing Outsourcing market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Service (Bioanalytical Testing, Method Development & Validation, Stability Testing, Other Services).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Bioanalytical Testing segment, which is expected to reach US$6.4 Billion by 2030 with a CAGR of a 8%. The Method Development & Validation segment is also set to grow at 6.8% CAGR over the analysis period.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pharmaceutical Analytical Testing Outsourcing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pharmaceutical Analytical Testing Outsourcing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pharmaceutical Analytical Testing Outsourcing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Bioreliance, Boston Analytical, Charles River Laboratories International, Inc., Dalton Pharma Services, Eurofins Scientific and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Pharmaceutical Analytical Testing Outsourcing market report include:
- Bioreliance
- Boston Analytical
- Charles River Laboratories International, Inc.
- Dalton Pharma Services
- Eurofins Scientific
- Exova
- Halo Pharma
- Intertek Group PLC
- Pace Analytical Services, LLC
- Pharmaceutical Product Development, LLC
- SGS SA
- Toxikon, Inc.
- West Pharmaceutical Services, Inc.
- WuXi AppTec, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Bioreliance
- Boston Analytical
- Charles River Laboratories International, Inc.
- Dalton Pharma Services
- Eurofins Scientific
- Exova
- Halo Pharma
- Intertek Group PLC
- Pace Analytical Services, LLC
- Pharmaceutical Product Development, LLC
- SGS SA
- Toxikon, Inc.
- West Pharmaceutical Services, Inc.
- WuXi AppTec, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 112 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
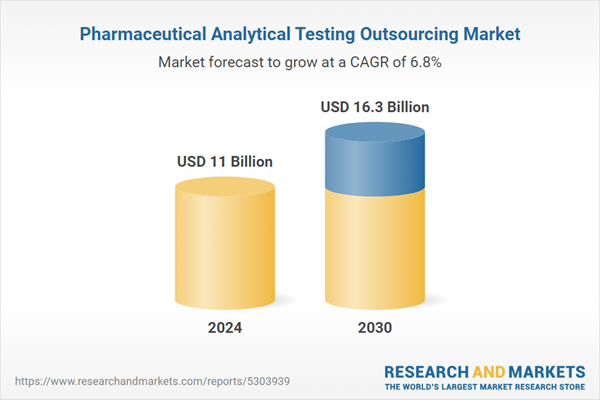
| Estimated Market Value ( USD | $ 11 Billion |
| Forecasted Market Value ( USD | $ 16.3 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |