The COVID-19 pandemic had a significant impact on the market studied in Australia. Many companies received approval for their vaccines against the Sars-CoV-2 virus and focused their research and development on therapeutics against COVID-19. For instance, in January 2021, the Therapeutic Goods Administration approved the COVID-19 vaccine developed by Pfizer and BioNTech. In addition, the Australian government signed nearly four agreements and invested around AUD 3.3 billion to support the supply of vaccines in the country. Furthermore, the country invested around AUD 363 million in research and development activities related to the prevention and treatment of COVID-19. Such developments accelerated market growth during a pandemic. Furthermore, the Australian Government has convened a large group of clinical experts to form the National COVID-19 Clinical Evidence Taskforce. The role of the taskforce is to create evidence-based Australian guidelines for the clinical care of people with COVID-19. The Therapeutic Goods Administration (TGA) approved Tixa Genmab and ciljevima (Evoshield) in December 2021 and Sotrovimab (Xevudy) in August 2021 for COVID-19 treatment. Hence, it is observed that the COVID-19 pandemic had a significant impact on the market studied.
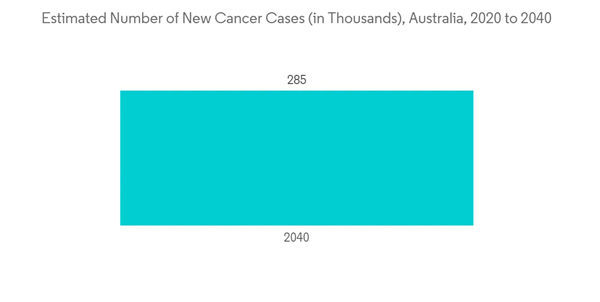
The major factors likely to drive market growth during the forecast period are the growing burden of chronic diseases and the rising geriatric population, along with the rising investments in research and development expenditure for novel therapeutics in Australia. According to Australian Bureau of Statistics data updated in March 2022, over three-quarters, i.e., 78.6% of Australians, had at least one long-term health condition in 2021, while nearly half, 46.6% or 11.6 million, had at least one chronic condition. Thus, increasing chronic diseases and a high demand for pharmaceutical products are expected to promote market growth. Furthermore, the burden of diabetes has also increased in the country. As per the data in the International Diabetes Federation’s 2021 report, there were about 1.5 million people with diabetes in Australia in 2021, and their number is expected to increase to 1.9 million by 2045. Thus, the demand for pharmaceuticals for diabetes treatment and management is expected to increase over the years in Australia, which is expected to boost growth in the pharmaceuticals market in the country.
Additionally, key strategies adopted by major market players, increased investment, and rising research and development activities will also contribute to market growth. For instance, in June 2022, diabetes-focused biotech player Dimerix entered into a partnership agreement with the Australian Centre for Accelerating Diabetes Innovations (ACADI) to conduct a clinical trial of its oral medical product DMX-200 in diabetic kidney disease patients. Positive results from such studies will boost their demand, hence boosting the pharmaceutical manufacturing process and thereby driving the market during the study period. Thus, the above-mentioned factors are expected to positively contribute to the market's growth over the study period.
However, strict regulatory guidelines for product approvals are expected to hinder market growth.
Australia Pharmaceuticals Market Trends
The Prescription Drugs Segment Holds the Largest Share and is Expected to do so in the Forecast Period
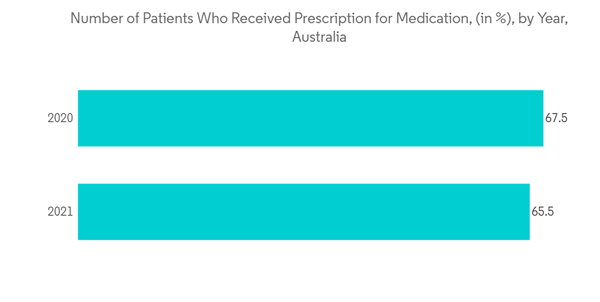
Prescription drugs are available with a valid prescription from a prescriber. These drugs are heavily regulated and require a visit to a doctor, a diagnosis, and monitoring by a doctor to ensure the medication is working and that it is working safely.According to the November 2021 report of the Australian Institute of Health and Welfare (AIHW), about 16% of the total Australian population was of age 65 years and more, and the older population is on the rise. The rising geriatric population is expected to fuel the market growth as they are more prone to various chronic diseases such as cancer, cardiovascular diseases, musculoskeletal diseases, and others. The increasing burden of chronic diseases is one of the key factors fueling the demand for therapeutic drugs which will boost the market. In addition, the launch of new drugs in the country requiring a doctor's prescription is expected to positively contribute to the segment's growth. For instance, in January 2022, Australia’s drug regulator approved two prescription drugs for the treatment of COVID-19, Lagevrio by Merck and Paxlovid from Pfizer.
Moreover, technological advancements in the segment with the purpose to ease the prescription process will also drive the market. For instance, in February 2022, the Australian Capital Territory Government launched Canberra Script, a real-time prescription monitoring system to enable medical professionals to assess patients’ drug history before prescribing and dispensing some medications. It is being implemented to help address the increasing harms caused by some prescription medications, particularly opioids.
Therefore, due to the aforementioned factors, the prescription drugs segment is expected to have a significant market share in the pharmaceutical market in Australia over the forecast period.
Generic Drugs are Expected to Witness Strong Growth in Coming Years
A generic drug is a medication created to be the same as an already marketed branded drug in dosage form, safety, strength, route of administration, quality, performance characteristics, and intended use.The use of generic drugs in Australia is continuously rising due to their cost-effectiveness and the presence of pharmaceutical companies that develop such generic medicines. Additionally, the key steps taken by the Australian government play a crucial role in the demand for generic medicine. For instance, in March 2022, the Indian and Australian governments signed an agreement through which Indian pharmaceutical companies will get easy access to the Australian market for launching their products. The Therapeutic Good Administration (TGA) of Australia agreed to expedite faster and easier approval for the drug developed by Indian pharmaceutical companies. With such agreements, the segment is expected to witness strong growth in the coming years.
Furthermore, new launches of generic drugs in Australia are accelerating segment growth. For instance, in March 2022, TiefenbacherPharmaceuticals successfully launched the first generic version of the Epilepsy medicine Lacosamide in Australia. Lacosamide is used for the treatment of seizures in patients suffering from epilepsy. The tablets are being marketed in Australia in the strengths 50mg, 100mg, 150mg, and 200mg. With the launch of such drugs for crucial diseases, the genetic drug demand is expected to witness a significant hike in the coming years across the country.
Australia Pharmaceuticals Market Competitor Analysis
The Australian pharmaceutical market is highly competitive and consists of several major players. In terms of market share, a few of the major players are currently dominating the market. Some prominent players are vigorously making acquisitions and joint ventures with other companies to consolidate their market positions in the country. Some of the key companies currently dominating the market are Abbvie Inc., Amgen Inc., Pfizer Inc., AstraZeneca, and Eli Lilly and Company.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- AstraZeneca PLC
- Amgen Inc.
- Pfizer Inc.
- CSL Limited
- F. Hoffmann-La Roche AG
- GlaxoSmithKline PLC
- Eli Lilly and Company
- Novartis AG
- Sanofi SA
- Johnson and Johnson
- Merck KGaA