Global Lysosomal Storage Diseases Market - Key Trends & Drivers Summarized
What Are Lysosomal Storage Diseases, and Why Do They Require Specialized Treatments?
Lysosomal Storage Diseases (LSDs) are a group of rare genetic disorders caused by enzyme deficiencies that prevent the normal breakdown of complex molecules within lysosomes, leading to toxic accumulation and cellular dysfunction. These disorders, which include Gaucher disease, Fabry disease, Pompe disease, and mucopolysaccharidoses (MPS), can result in severe complications affecting multiple organ systems, including the nervous system, skeletal structure, liver, and cardiovascular system. Due to their progressive nature, LSDs often require early diagnosis and long-term management, making them a focus area for rare disease research and pharmaceutical development. The increasing availability of newborn screening programs and advancements in genetic testing have significantly improved early detection rates, enabling timely intervention and better patient outcomes.The treatment landscape for LSDs has evolved significantly, with enzyme replacement therapy (ERT) emerging as the standard of care for several LSDs, including Gaucher and Fabry disease. ERT helps restore deficient enzyme activity, reducing disease progression and improving quality of life. However, challenges such as high treatment costs, limited therapeutic efficacy in crossing the blood-brain barrier (for neurological LSDs), and patient access barriers have driven the development of alternative therapies. Gene therapy and substrate reduction therapy (SRT) are gaining traction as promising approaches to address the underlying genetic causes of LSDs, offering potential long-term solutions. As pharmaceutical companies invest in novel treatment modalities and precision medicine strategies, the LSD market continues to expand, aiming to improve both efficacy and accessibility for affected individuals.
What Are the Key Innovations and Research Advancements in LSD Treatments?
Significant strides in biotechnology and gene therapy have fueled innovation in the LSD market, leading to the development of next-generation therapeutics. One of the most promising advancements is gene therapy, which aims to deliver functional copies of defective genes directly to patient cells, potentially offering a one-time cure for certain LSDs. Several gene therapy candidates, particularly for Pompe disease and MPS disorders, are currently in clinical trials, with early data showing encouraging results. Additionally, stem cell transplantation and chaperone therapy, which stabilizes deficient enzymes, are being explored as adjunctive or alternative treatment options for LSD patients.Another critical area of advancement is blood-brain barrier (BBB) penetration strategies, as many LSDs cause severe neurological complications. Researchers are developing innovative drug delivery mechanisms, including nanoparticles and viral vector-based systems, to enhance the central nervous system uptake of therapies. Advances in CRISPR-based genome editing also hold potential for addressing the root causes of LSDs, potentially leading to permanent genetic corrections. As pharmaceutical companies and biotech firms intensify research efforts, regulatory agencies are providing expedited pathways, such as orphan drug designations and priority review programs, to accelerate the development and commercialization of LSD therapies.
Which Factors Are Driving the Demand for Lysosomal Storage Disease Treatments?
The increasing prevalence of rare genetic disorders, improved diagnostic capabilities, and growing patient advocacy efforts have fueled demand for LSD treatments. Advances in next-generation sequencing (NGS) and biomarker-based diagnostic tools have significantly enhanced early detection rates, leading to improved patient outcomes. The expansion of newborn screening programs in several countries has further contributed to early intervention efforts, ensuring timely access to treatment for infants diagnosed with LSDs.Additionally, increasing investments in rare disease research by pharmaceutical and biotech companies have led to a surge in drug development activities. The global rare disease market has witnessed a rise in regulatory incentives, including orphan drug exclusivity and fast-track approvals, encouraging more companies to enter the LSD treatment space. Patient advocacy groups and non-profit organizations are also playing a crucial role in driving awareness, funding research initiatives, and facilitating access to novel therapies. As the global healthcare ecosystem continues to prioritize rare diseases, LSD treatment options are expected to expand, improving both the quality of life and survival rates of affected individuals.
What Is Driving the Growth of the Lysosomal Storage Diseases Market?
The growth in the lysosomal storage diseases market is driven by several factors, including advancements in gene therapy, increasing regulatory approvals for novel treatments, and rising investments in orphan drug development. The emergence of gene-editing technologies and targeted enzyme therapies has significantly expanded treatment possibilities, offering hope for long-term disease management and potential cures. Pharmaceutical companies are actively investing in LSD research, with multiple late-stage clinical trials progressing toward commercialization.Government policies supporting rare disease drug development, along with financial incentives for orphan drugs, have further stimulated market expansion. Additionally, the growing integration of AI-driven drug discovery platforms has accelerated research efforts, leading to faster identification of viable therapeutic candidates. The expansion of healthcare infrastructure and specialized treatment centers has also improved patient access to LSD therapies, particularly in emerging markets. As innovations in genetic medicine continue to reshape the treatment landscape, the LSD market is poised for significant growth, offering new opportunities for both patients and stakeholders in the rare disease ecosystem.
Report Scope
The report analyzes the Lysosomal Storage Diseases market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Therapy (Enzyme Replacement Therapy (ERT), Substrate Reduction Therapy (SRT), Cystine Depleting Agents).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Enzyme Replacement Therapy (ERT) segment, which is expected to reach US$8.1 Billion by 2030 with a CAGR of 6%. The Substrate Reduction Therapy (SRT) segment is also set to grow at 5.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.4 Billion in 2024, and China, forecasted to grow at an impressive 5.3% CAGR to reach $2.6 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Lysosomal Storage Diseases Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Lysosomal Storage Diseases Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Lysosomal Storage Diseases Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alexion Pharmaceuticals, Inc., BioMarin Pharmaceutical, Inc., Horizon Therapeutics plc, Lacerta Therapeutics, Orphazyme ApS and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Lysosomal Storage Diseases market report include:
- Alexion Pharmaceuticals, Inc.
- BioMarin Pharmaceutical, Inc.
- Horizon Therapeutics plc
- Lacerta Therapeutics
- Orphazyme ApS
- Oxyrane
- Sanofi Genzyme
- Sanofi SA
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alexion Pharmaceuticals, Inc.
- BioMarin Pharmaceutical, Inc.
- Horizon Therapeutics plc
- Lacerta Therapeutics
- Orphazyme ApS
- Oxyrane
- Sanofi Genzyme
- Sanofi SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
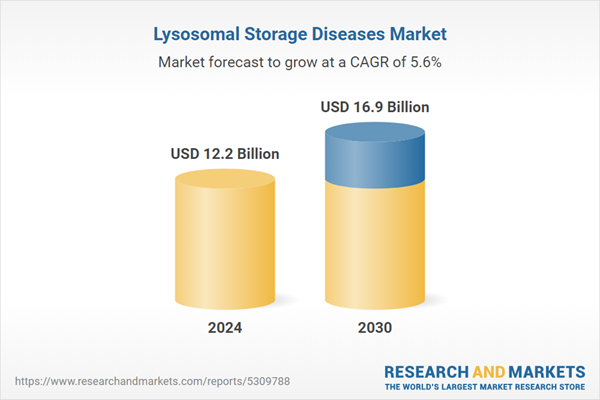
| Estimated Market Value ( USD | $ 12.2 Billion |
| Forecasted Market Value ( USD | $ 16.9 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |