Global Renal Biomarkers Market - Key Trends and Drivers Summarized
Renal Biomarkers: Advancing Kidney Disease Diagnosis and Management
Renal biomarkers are essential tools in the early detection, diagnosis, and management of kidney diseases. These biomarkers are transforming the diagnosis and management of kidney diseases by providing more accurate, timely, and non-invasive methods for detecting renal dysfunction. These biomarkers are measurable indicators present in blood, urine, or tissue samples that reflect the functional state of the kidneys. These biomarkers are categorized into various types based on their biological functions and roles in renal pathophysiology. Functional biomarkers, such as creatinine and blood urea nitrogen (BUN), are widely used to assess kidney function and detect any deviation from normal physiological processes. These markers are typically measured to monitor the glomerular filtration rate (GFR), which is crucial in diagnosing conditions like acute kidney injury (AKI) and chronic kidney disease (CKD). Up-regulated proteins, including neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1), are indicative of kidney stress or damage. These proteins are typically expressed at higher levels when the kidneys are under stress or have suffered injury, making them valuable in detecting early-stage kidney damage before significant functional decline. Other biomarkers, such as cystatin C and beta-2 microglobulin, offer additional insights into kidney health, providing a more comprehensive understanding of renal function and pathology.Diagnostic techniques for detecting and quantifying renal biomarkers are critical for accurate and timely diagnosis. Enzyme-linked immunosorbent assays (ELISAs) are widely used due to their sensitivity and specificity in detecting low concentrations of biomarkers. ELISAs are particularly useful in routine clinical settings for the quantification of proteins such as NGAL and KIM-1. Particle-enhanced turbidimetric immunoassays (PETIA) offer an alternative approach, especially for the measurement of functional biomarkers like cystatin C, providing rapid and reliable results with minimal sample preparation. Colorimetric assays, though less specific, are commonly employed for measuring creatinine and BUN, offering a quick and cost-effective method for routine kidney function tests. Advanced techniques like liquid chromatography-mass spectrometry (LC-MS) provide highly accurate and detailed profiling of multiple biomarkers simultaneously, making them invaluable in research settings and for the development of new diagnostic tests. Chemiluminescent enzyme immunoassays (CLIA) combine the sensitivity of ELISAs with enhanced detection capabilities, making them suitable for high-throughput testing environments where rapid and precise results are required.
What Technological Advancements Are Enhancing Renal Biomarker Detection?
Technological advancements are significantly enhancing the detection and analysis of renal biomarkers, driving the adoption of these tools in clinical practice. One of the key trends is the development of high-sensitivity assays and point-of-care diagnostic devices that enable rapid and accurate measurement of renal biomarkers in clinical settings. These advancements allow for timely diagnosis and monitoring of kidney disease, reducing the need for invasive procedures and improving patient comfort. The integration of artificial intelligence and machine learning in biomarker analysis is also gaining traction, enabling more precise interpretation of biomarker data and the identification of complex patterns associated with renal disease progression. Additionally, advancements in multiplexing technologies are allowing for the simultaneous measurement of multiple biomarkers, providing a more comprehensive assessment of kidney function and facilitating personalized treatment approaches. These technological innovations are driving the widespread adoption of renal biomarkers in nephrology, supporting more effective diagnosis and management of kidney diseases.What Are the Key Applications and Benefits of Renal Biomarkers?
Renal biomarkers are used in a variety of clinical applications, offering significant benefits that enhance the diagnosis, management, and treatment of kidney diseases. In early diagnosis, biomarkers such as NGAL and cystatin C provide valuable information that can detect acute kidney injury (AKI) before significant damage occurs, allowing for prompt intervention and potentially preventing the progression to chronic kidney disease (CKD). In disease monitoring, renal biomarkers help clinicians assess the effectiveness of treatment and make informed decisions about therapy adjustments, improving patient outcomes. Biomarkers are also critical in the stratification of patients for clinical trials, enabling the identification of individuals who are most likely to benefit from specific interventions. The primary benefits of renal biomarkers include earlier detection of kidney disease, improved treatment outcomes, reduced healthcare costs, and the potential for personalized medicine approaches. These advantages make renal biomarkers an essential tool in modern nephrology, supporting better care for patients with kidney diseases.The end-use of renal biomarkers spans across various healthcare settings, including diagnostic laboratories, hospitals, and other specialized facilities. Diagnostic laboratories play a pivotal role in processing and analyzing samples for renal biomarkers, utilizing a range of techniques from basic colorimetric assays to advanced LC-MS. These laboratories are essential for providing accurate and timely results, which are crucial for the effective management of kidney diseases. Hospitals, on the other hand, use renal biomarkers as part of routine diagnostic workups and monitoring of patients with acute or chronic kidney conditions. In emergency settings, rapid tests for biomarkers like creatinine or NGAL can be life-saving, providing critical information that guides immediate treatment decisions. Other end-uses include research institutions and pharmaceutical companies, where renal biomarkers are used to study disease mechanisms, evaluate the efficacy of new treatments, and identify potential therapeutic targets.
What Factors Are Driving the Growth in the Renal Biomarkers Market?
The growth in the renal biomarkers market is driven by several factors, including the increasing prevalence of kidney diseases worldwide, which has heightened the need for early detection and precise monitoring. The rising incidence of diabetes and hypertension, major risk factors for kidney disease, is also contributing to the demand for reliable biomarkers. Technological advancements in diagnostic techniques, such as the development of more sensitive and specific assays, are enabling earlier and more accurate detection of kidney injuries and dysfunctions, thus expanding the market. Additionally, the shift towards personalized medicine, where treatments are tailored based on individual biomarker profiles, is further boosting the adoption of renal biomarkers. The growing focus on preventive healthcare, coupled with the increasing awareness of kidney health, is driving demand in both clinical and research settings, ensuring robust growth in the renal biomarkers market in the coming years.Report Scope
The report analyzes the Renal Biomarkers market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Biomarker (Functional Biomarkers, Up-Regulated Proteins, Other Biomarkers); Diagnostic Technique (Enzyme-Linked Immunosorbent Assays, Particle-Enhanced Turbidimetric Immunoassays (PETIA), Colorimetric Assays, Liquid Chromatography Mass Spectrometry (LS-MS), Chemiluminescent Enzyme Immunoassays (CLIA)); End-Use (Diagnostic Laboratories, Hospitals, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Functional Biomarkers segment, which is expected to reach US$1.1 Billion by 2030 with a CAGR of 7%. The Up-Regulated Proteins segment is also set to grow at 6.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $376.4 Million in 2024, and China, forecasted to grow at an impressive 6.1% CAGR to reach $311 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Renal Biomarkers Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Renal Biomarkers Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Renal Biomarkers Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Bioporto Diagnostics A/S, GenXPro GmbH, Helier Scientific Ltd., ViroGates A/S, Yaxon Biocare Pvt. Ltd and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Renal Biomarkers market report include:
- Bioporto Diagnostics A/S
- GenXPro GmbH
- Helier Scientific Ltd.
- ViroGates A/S
- Yaxon Biocare Pvt. Ltd
- Zeta Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Bioporto Diagnostics A/S
- GenXPro GmbH
- Helier Scientific Ltd.
- ViroGates A/S
- Yaxon Biocare Pvt. Ltd
- Zeta Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | February 2026 |
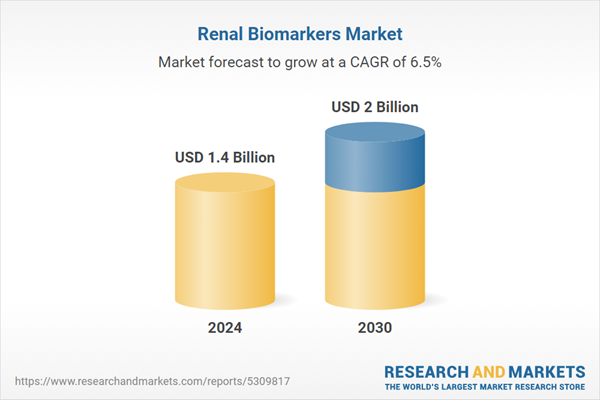
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.4 Billion |
| Forecasted Market Value ( USD | $ 2 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |