Global Subcutaneous Immunoglobulin Market - Key Trends & Drivers Summarized
What Is Subcutaneous Immunoglobulin and Why Is It Crucial for Immunodeficiency Treatment?
Subcutaneous Immunoglobulin (SCIg) is a therapeutic preparation of immunoglobulins administered through the subcutaneous route, typically used for the treatment of primary immunodeficiency diseases (PIDs), secondary immunodeficiencies, and certain autoimmune disorders. SCIg provides the body with a concentrated dose of antibodies, derived from pooled human plasma, to help strengthen the immune system's response to infections and other diseases. This form of immunoglobulin therapy is designed for regular administration, usually weekly or biweekly, allowing patients to maintain stable antibody levels and reduce the risk of recurrent infections. SCIg is often preferred over intravenous immunoglobulin (IVIg) due to its ease of use, reduced risk of systemic side effects, and the potential for home-based administration, which enhances patient comfort and compliance.The global adoption of SCIg is driven by its efficacy in providing long-term immune support and its ability to improve the quality of life for individuals with chronic immunodeficiency conditions. Primary immunodeficiency diseases, such as common variable immunodeficiency (CVID) and X-linked agammaglobulinemia (XLA), impair the body's ability to produce adequate antibodies, making patients susceptible to frequent and severe infections. SCIg therapy compensates for this deficiency by delivering a steady supply of antibodies that help protect against bacterial, viral, and fungal pathogens. Additionally, SCIg is increasingly being used to treat secondary immunodeficiencies caused by underlying diseases or immunosuppressive therapies, such as in patients undergoing chemotherapy or those with HIV. The rising prevalence of immunodeficiency disorders, coupled with the growing demand for convenient and effective treatment options, is driving the growth of the SCIg market as healthcare providers seek to improve patient outcomes and reduce the burden of disease.
How Are Technological Advancements Shaping the Development and Delivery of Subcutaneous Immunoglobulin Therapies?
Technological advancements are significantly enhancing the development, formulation, and delivery of subcutaneous immunoglobulin therapies, making them more effective, user-friendly, and accessible for a broader patient population. One of the most impactful innovations in this field is the development of advanced SCIg formulations with improved stability, concentration, and tolerability. Modern SCIg products are being designed with higher protein concentrations, allowing for lower injection volumes and shorter infusion times, which enhance patient convenience and adherence to treatment regimens. The use of stabilizing agents, such as sugars or amino acids, in SCIg formulations is improving the stability and shelf life of these products, ensuring consistent therapeutic efficacy. Additionally, the development of hypoallergenic SCIg formulations with reduced levels of IgA and other potential immunogenic components is minimizing the risk of adverse reactions, making SCIg therapy safer for patients with sensitivities or allergies to certain plasma proteins.Another key technological advancement driving the SCIg market is the innovation in infusion devices and delivery systems. Traditional SCIg administration involved the use of manual syringes or infusion pumps, which could be cumbersome and time-consuming for patients. Modern SCIg therapies are now available with advanced infusion devices, such as wearable pumps and needle-free injectors, that simplify the administration process and allow for more precise control over infusion rates. These devices are often compact, portable, and easy to use, enabling patients to self-administer SCIg at home with minimal assistance. Wearable devices, such as on-body pumps, allow for continuous or controlled-rate infusion, reducing the frequency of infusions and improving patient comfort. Additionally, innovations in needle technology, such as the development of ultra-fine needles and multi-needle sets, are minimizing injection pain and discomfort, enhancing the overall patient experience. These advancements are making SCIg therapy more accessible and convenient, supporting its adoption in home-based care settings and improving patient quality of life.
Furthermore, advancements in digital health and remote monitoring technologies are enhancing the management and delivery of SCIg therapy. Digital health tools, such as mobile apps and connected devices, are being integrated with SCIg delivery systems to provide real-time monitoring of infusion parameters, track treatment adherence, and offer personalized support to patients. These tools allow healthcare providers to remotely monitor patients' progress, identify potential issues, and adjust treatment plans as needed, reducing the need for frequent clinic visits. The use of telemedicine and virtual consultations is further supporting the adoption of home-based SCIg therapy by providing patients with access to specialist care and guidance from the comfort of their homes. These technological advancements are improving the safety, efficiency, and patient-centeredness of SCIg therapy, making it a more attractive option for individuals with chronic immunodeficiency conditions.
What Factors Are Driving the Adoption of Subcutaneous Immunoglobulin Therapy Across Different Patient Populations and Regions?
The adoption of subcutaneous immunoglobulin therapy is being driven by several key factors, including the growing prevalence of primary and secondary immunodeficiencies, the increasing awareness and diagnosis of immunodeficiency disorders, and the rising preference for home-based treatment options. One of the primary drivers is the growing prevalence of primary and secondary immunodeficiencies worldwide. Primary immunodeficiencies are a group of over 400 rare, inherited disorders that affect the immune system's ability to function properly. The increasing recognition and diagnosis of these conditions, along with improvements in genetic testing and screening, are contributing to a larger patient population eligible for immunoglobulin replacement therapy. Similarly, secondary immunodeficiencies, which can result from medical treatments such as chemotherapy, organ transplantation, or chronic infections, are becoming more common due to the rising incidence of cancer and autoimmune diseases. SCIg therapy is being adopted as an effective treatment option for these patients, providing them with a continuous supply of antibodies to support immune function and reduce the risk of infections.Another significant factor driving the adoption of SCIg therapy is the increasing awareness and diagnosis of immunodeficiency disorders. Greater awareness among healthcare providers, patients, and caregivers about the signs and symptoms of immunodeficiencies is leading to earlier diagnosis and timely intervention with SCIg therapy. Patient advocacy organizations, such as the Immune Deficiency Foundation (IDF) and the International Patient Organization for Primary Immunodeficiencies (IPOPI), are playing a crucial role in educating the public and promoting early detection and treatment. The availability of comprehensive treatment guidelines and consensus statements from medical societies is also supporting the adoption of SCIg as a standard of care for eligible patients. This growing awareness is particularly strong in developed regions such as North America and Europe, where healthcare systems are well-equipped to diagnose and manage rare and complex immunological conditions. As more patients receive accurate diagnoses and are educated about their treatment options, the demand for SCIg therapy is expected to rise, supporting the growth of the market.
Moreover, the rising preference for home-based treatment options is influencing the adoption of SCIg therapy across various regions. Unlike intravenous immunoglobulin (IVIg), which is typically administered in a hospital or clinical setting, SCIg can be self-administered by patients or caregivers at home, offering greater flexibility and convenience. Home-based SCIg therapy reduces the need for frequent clinic visits, minimizes the risk of hospital-acquired infections, and allows patients to integrate treatment into their daily routines. This home-based approach is particularly beneficial for pediatric patients, elderly patients, and those with mobility issues, as it reduces the physical and logistical burden associated with frequent travel to healthcare facilities. The COVID-19 pandemic has further accelerated the adoption of home-based therapies, as patients and healthcare providers seek to minimize in-person interactions and reduce exposure risks. The growing acceptance of home-based SCIg therapy is driving demand for user-friendly delivery systems and digital health tools that support patient self-management and improve adherence.
What Is Driving the Growth of the Global Subcutaneous Immunoglobulin Market?
The growth in the global Subcutaneous Immunoglobulin (SCIg) market is driven by several factors, including rising investments in research and development (R&D), the increasing adoption of advanced SCIg delivery systems, and the growing focus on personalized medicine and patient-centered care. One of the primary growth drivers is the rising investment in R&D activities aimed at developing novel SCIg formulations and delivery devices. Leading biopharmaceutical companies are investing heavily in the development of high-concentration SCIg products, long-acting formulations, and novel stabilizing agents that enhance the safety and efficacy of SCIg therapy. The focus on R&D is also leading to the introduction of new indications for SCIg, such as its use in chronic inflammatory demyelinating polyneuropathy (CIDP) and other neurological conditions, expanding the therapeutic applications of SCIg beyond immunodeficiency. Clinical trials and post-marketing studies are providing evidence of the benefits of SCIg therapy in diverse patient populations, supporting its adoption as a first-line treatment option for eligible patients. These R&D efforts are supporting the growth of the SCIg market by providing patients and healthcare providers with innovative treatment options that improve outcomes and quality of life.Another significant driver of market growth is the increasing adoption of advanced SCIg delivery systems. The development of user-friendly, portable, and wearable SCIg infusion devices is making it easier for patients to self-administer therapy at home. Devices such as wearable pumps, smart infusion systems, and needle-free injectors are simplifying the administration process and allowing for more precise control over infusion parameters. The use of electronic and smart devices that provide feedback, track infusion progress, and alert patients or caregivers in case of issues is further enhancing the safety and convenience of SCIg therapy. These advanced delivery systems are reducing the learning curve associated with SCIg administration and making it more accessible to a wider patient population, including those with limited experience in self-care. The increasing availability of these devices, along with the expansion of home healthcare services, is driving the adoption of SCIg therapy and supporting the growth of the market.
Moreover, the growing focus on personalized medicine and patient-centered care is supporting the growth of the SCIg market. Personalized medicine involves tailoring treatment plans to the individual needs and preferences of each patient, taking into account factors such as disease severity, lifestyle, and response to therapy. SCIg therapy, with its flexibility in dosing and administration schedules, is well-suited for personalized treatment approaches. Healthcare providers can adjust the dose, frequency, and site of administration based on the patient's clinical response and preferences, optimizing therapeutic outcomes. The use of digital health tools, such as mobile apps and connected devices, is further enabling personalized care by providing real-time data on treatment adherence, patient-reported outcomes, and infusion parameters. These tools allow for more precise and individualized treatment management, supporting better patient engagement and satisfaction. The growing emphasis on personalized medicine is driving demand for SCIg therapies that offer flexibility, convenience, and patient-centeredness, supporting the expansion of the market.
Furthermore, the increasing demand for safe and effective alternatives to IVIg therapy is influencing the growth of the SCIg market. While IVIg remains a standard treatment for many immunodeficiency and autoimmune conditions, it requires intravenous access and close monitoring, which can be challenging for some patients. SCIg offers a more convenient alternative that can be administered at home with a lower risk of systemic side effects, such as headaches, fever, and infusion-related reactions. The development of high-concentration SCIg products is allowing for reduced infusion volumes and shorter administration times, making SCIg a viable option for patients who prefer less invasive and time-consuming therapies. The increasing preference for SCIg over IVIg is driving the growth of the SCIg market, as patients and healthcare providers seek treatment options that offer improved quality of life and ease of use. As these factors continue to shape the global healthcare landscape, the Subcutaneous Immunoglobulin market is expected to experience robust growth, driven by rising investments in R&D, the increasing adoption of advanced SCIg delivery systems, and the growing focus on personalized medicine and patient-centered care.
Report Scope
The report analyzes the Subcutaneous Immunoglobulin market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Application (Primary Immunodeficiency Diseases, Secondary Immunodeficiency Diseases); End-Use (Hospitals, Clinics, Homecare Settings).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Subcutaneous Immunoglobulin Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Subcutaneous Immunoglobulin Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Subcutaneous Immunoglobulin Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Biotest AG, CSL Behring, Grifols International SA, Grifols SA, Kedrion SpA and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 46 companies featured in this Subcutaneous Immunoglobulin market report include:
- Biotest AG
- CSL Behring
- Grifols International SA
- Grifols SA
- Kedrion SpA
- Octapharma AG
- Shire PLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Biotest AG
- CSL Behring
- Grifols International SA
- Grifols SA
- Kedrion SpA
- Octapharma AG
- Shire PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
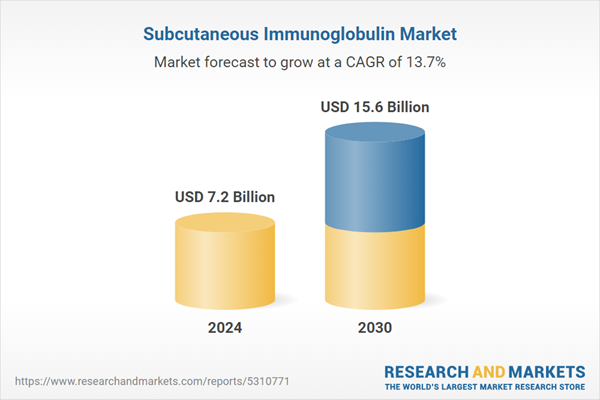
| Estimated Market Value ( USD | $ 7.2 Billion |
| Forecasted Market Value ( USD | $ 15.6 Billion |
| Compound Annual Growth Rate | 13.7% |
| Regions Covered | Global |