Global Scleroderma Diagnostics and Therapeutics Market - Key Trends & Drivers Summarized
What Is Scleroderma and Why Is It a Growing Focus for Diagnostics and Therapeutics?
Scleroderma, also known as systemic sclerosis, is a chronic autoimmune disease characterized by excessive collagen deposition in the skin and internal organs, leading to fibrosis, vascular abnormalities, and immune system dysregulation. The disease manifests in two primary forms: localized scleroderma, which affects only the skin and subcutaneous tissues, and systemic scleroderma, which can impact multiple organs such as the lungs, heart, kidneys, and gastrointestinal tract. Patients with scleroderma often experience a range of symptoms, including skin thickening, joint pain, Raynaud's phenomenon, and respiratory complications, which can severely impact quality of life and lead to life-threatening complications. Diagnosing scleroderma is challenging due to its heterogeneous presentation and overlapping symptoms with other autoimmune diseases. Therefore, early and accurate diagnosis is critical to managing the disease effectively and preventing irreversible organ damage.The increasing focus on scleroderma diagnostics and therapeutics is driven by the rising prevalence of autoimmune diseases globally and the unmet medical need for effective treatment options. Currently, there is no cure for scleroderma, and available therapies primarily aim to alleviate symptoms, manage complications, and slow disease progression. Early diagnosis and intervention are crucial for improving patient outcomes, making advancements in diagnostic techniques a key priority. The standard diagnostic approach for scleroderma includes clinical evaluation, laboratory testing for specific autoantibodies (such as anti-nuclear antibodies, anti-centromere antibodies, and anti-topoisomerase antibodies), and imaging studies like high-resolution CT scans and echocardiography. However, ongoing research into novel biomarkers and non-invasive diagnostic methods is paving the way for more accurate and earlier detection of scleroderma, which is essential for initiating timely treatment. As awareness of the disease grows and research efforts intensify, the scleroderma diagnostics and therapeutics market is poised for expansion, driven by the need for better diagnostic tools and more effective therapeutic options.
How Are Technological Advancements Shaping the Diagnosis and Treatment of Scleroderma?
Technological advancements are significantly transforming the landscape of scleroderma diagnosis and treatment, enabling earlier detection, more accurate assessment of disease activity, and the development of targeted therapies. One of the most notable advancements in diagnostics is the identification of novel biomarkers that can serve as early indicators of disease onset and progression. Researchers are exploring the role of various autoantibodies, cytokines, and genetic markers in scleroderma pathogenesis, which could lead to the development of more sensitive and specific diagnostic tests. For instance, the detection of specific autoantibodies such as anti-RNA polymerase III and anti-fibrillin-1 can provide valuable insights into disease subtypes and prognosis. Additionally, advances in imaging technologies, such as high-resolution ultrasound and magnetic resonance imaging (MRI), are improving the ability to assess skin and organ involvement non-invasively. These imaging modalities are being used to monitor disease progression and evaluate treatment responses, offering a more comprehensive approach to disease management.In the therapeutics domain, innovations are driving the development of targeted therapies that address the underlying mechanisms of scleroderma rather than just managing symptoms. Traditional treatment options, such as immunosuppressants, corticosteroids, and vasodilators, have limitations in terms of efficacy and safety. Newer therapeutic approaches, including biologics and small molecule inhibitors, are showing promise in clinical trials. For example, targeted biologic agents like tocilizumab (an IL-6 receptor antagonist) and rituximab (a B-cell depleting agent) have demonstrated efficacy in reducing inflammation and fibrosis in scleroderma patients. Additionally, antifibrotic therapies, such as nintedanib and pirfenidone, which are used in idiopathic pulmonary fibrosis, are being investigated for their potential to treat scleroderma-associated interstitial lung disease (SSc-ILD), a leading cause of morbidity and mortality in scleroderma patients. The application of precision medicine and pharmacogenomics is also contributing to the development of personalized treatment strategies based on an individual's genetic and molecular profile, enhancing therapeutic efficacy and reducing adverse effects. These technological advancements are not only improving diagnostic accuracy and therapeutic outcomes but are also paving the way for a more personalized approach to scleroderma management.
What Factors Are Driving the Adoption of Advanced Diagnostics and Therapeutics for Scleroderma?
The adoption of advanced diagnostics and therapeutics for scleroderma is being driven by several key factors, including the rising prevalence of autoimmune diseases, increasing awareness of scleroderma among healthcare providers and patients, and the expanding research into novel therapeutic targets. One of the primary drivers is the growing incidence of autoimmune diseases worldwide, which has led to a higher prevalence of scleroderma and other related conditions. This trend is prompting healthcare providers to adopt advanced diagnostic tools that can detect scleroderma at an earlier stage and differentiate it from other autoimmune disorders. Early and accurate diagnosis is essential for initiating appropriate treatment and preventing severe complications. The use of advanced serological testing, coupled with imaging technologies like nailfold capillaroscopy and lung function tests, is enabling clinicians to identify scleroderma earlier, improving patient outcomes and reducing disease burden.Another significant factor contributing to the increased adoption of advanced scleroderma diagnostics and therapeutics is the growing awareness of the disease and its complications. Patient advocacy groups and professional organizations, such as the Scleroderma Foundation and the European League Against Rheumatism (EULAR), are playing a crucial role in raising awareness, promoting research, and providing education to both patients and healthcare providers. These initiatives are driving the demand for better diagnostic tools and treatment options by highlighting the need for early intervention and comprehensive care. Additionally, the increasing investment in research and development by pharmaceutical companies and academic institutions is accelerating the discovery of novel therapeutic targets. This is leading to the development of innovative therapies, such as biologics, antifibrotic agents, and gene therapies, that have the potential to modify the course of the disease rather than just manage symptoms. The success of these therapies in clinical trials is encouraging their adoption in clinical practice, providing patients with new hope for more effective treatment options. As these factors continue to influence the market, the adoption of advanced scleroderma diagnostics and therapeutics is expected to grow, offering improved outcomes for patients worldwide.
What Is Driving the Growth of the Global Scleroderma Diagnostics and Therapeutics Market?
The growth in the global Scleroderma Diagnostics and Therapeutics market is driven by several factors, including advancements in diagnostic technologies, the development of novel therapies, and increasing healthcare expenditures. One of the primary growth drivers is the continuous advancement in diagnostic tools and techniques. The ability to detect disease-specific biomarkers, coupled with improvements in imaging modalities, is enabling earlier and more accurate diagnosis of scleroderma. Early diagnosis is critical for initiating timely treatment and preventing complications, making advanced diagnostic technologies a key growth driver for the market. The development of companion diagnostics, which are used alongside specific therapies to optimize treatment selection and dosing, is also contributing to market growth by facilitating a more personalized approach to disease management.The introduction of novel therapies and targeted treatment options is another major driver of market growth. The scleroderma therapeutics landscape is evolving rapidly, with several new drug candidates showing promise in clinical trials. These include biologics, small molecule inhibitors, and antifibrotic agents that target the underlying pathophysiological mechanisms of scleroderma. The success of these therapies in reducing disease activity and improving patient outcomes is driving their adoption in clinical practice, leading to increased market growth. Additionally, the expanding pipeline of investigational drugs and ongoing clinical trials are expected to bring new and innovative treatment options to the market in the coming years. Furthermore, rising healthcare expenditures, particularly in developed markets such as North America and Europe, are enabling greater access to advanced diagnostic and therapeutic solutions. Governments and healthcare organizations are investing in improving the infrastructure for diagnosing and managing complex autoimmune diseases, further supporting market expansion.
Moreover, the growing focus on patient-centric care and the integration of digital health technologies are contributing to the growth of the scleroderma diagnostics and therapeutics market. Digital health tools, such as telemedicine platforms and wearable devices, are enhancing patient monitoring and facilitating remote consultations, making it easier for patients to access specialist care and stay connected with their healthcare providers. This is particularly important for managing chronic conditions like scleroderma, where ongoing monitoring and adjustment of treatment plans are crucial. As these trends continue to shape the healthcare landscape, the global Scleroderma Diagnostics and Therapeutics market is poised for sustained growth, driven by technological advancements, increased research investment, and a strong emphasis on early diagnosis and personalized treatment strategies.
Report Scope
The report analyzes the Scleroderma Diagnostics and Therapeutics market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Disease Type (Localized, Systemic); Drug Class (Immunosuppressive Agents, Endothelin Receptor Antagonists, Other Drug Classes).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Localized Disease segment, which is expected to reach US$1.9 Billion by 2030 with a CAGR of a 4.5%. The Systemic Disease segment is also set to grow at 3.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $660.9 Million in 2024, and China, forecasted to grow at an impressive 3.8% CAGR to reach $486.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Scleroderma Diagnostics and Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Scleroderma Diagnostics and Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Scleroderma Diagnostics and Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Apollo Diagnostics, AstraZeneca Plc, aTyr Pharma, Inc., Bayer AG, Boehringer Ingelheim International GmbH and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Scleroderma Diagnostics and Therapeutics market report include:
- Apollo Diagnostics
- AstraZeneca Plc
- aTyr Pharma, Inc.
- Bayer AG
- Boehringer Ingelheim International GmbH
- Cabaletta Bio, Inc.
- Certa Therapeutics
- Chemomab
- Corbus Pharmaceuticals Holdings, Inc.
- F. Hoffmann-La Roche AG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Apollo Diagnostics
- AstraZeneca Plc
- aTyr Pharma, Inc.
- Bayer AG
- Boehringer Ingelheim International GmbH
- Cabaletta Bio, Inc.
- Certa Therapeutics
- Chemomab
- Corbus Pharmaceuticals Holdings, Inc.
- F. Hoffmann-La Roche AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
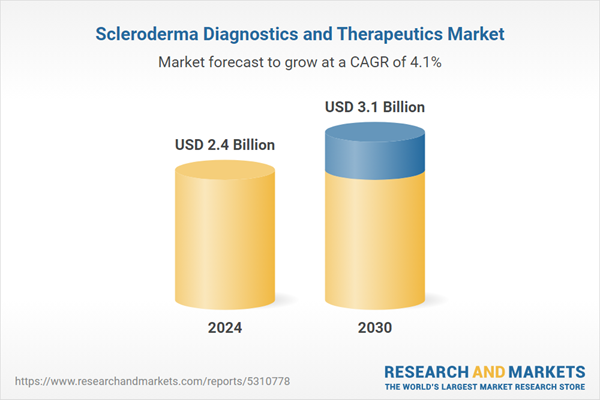
| Estimated Market Value ( USD | $ 2.4 Billion |
| Forecasted Market Value ( USD | $ 3.1 Billion |
| Compound Annual Growth Rate | 4.1% |
| Regions Covered | Global |