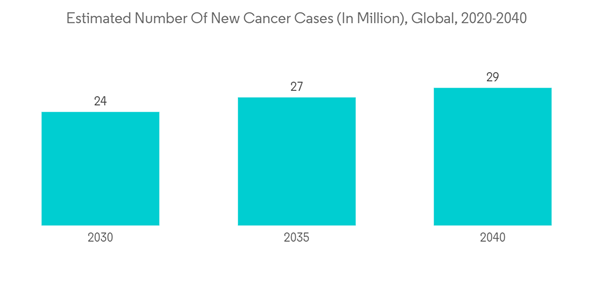COVID-19 severely impacted the generic drugs market during the early pandemic due to lockdown restrictions and supply chain disruption. Later, there was an increased demand for generic pharmaceuticals as the COVID-19 infections provided many opportunities for generic drug manufacturers to manufacture the drugs to treat this infection. As the COVID-19 public health emergency unfolded last year, the FDA shifted its focus to generic drug submissions involving potential treatments and supportive therapies for COVID-19 patients. To combat the virus's effects on patients, generic medicines such as intravenous drugs for patients on ventilators and steroids were used, which helped reduce COVID-19 fatalities. For instance, according to a report by the USFDA published in November 2021, the FDA has implemented a generic drug program. This initiative assisted generic medication developers with product development by sending written communications and holding meetings to clarify regulatory expectations early in the development process and during application review. The FDA received 121 requests for product development and pre-submission pre-abbreviated NDA meetings in 2020. Thus, such initiatives have positively impacted market growth in the post-pandemic phase. Thus, as per the analysis, the market studied is expected to follow the same trend over the forecast period as the demand for generic drugs is expected to increase.
Furthermore, the reasons attributed to the growth of the market are the increasing prevalence of chronic diseases, a surge in the geriatric population, and growing healthcare expenditure. As per the 2020 report of the International Agency for Research on Cancer (IARC), which estimated the incidence and mortality of 36 cancers in 185 countries globally, an estimated 19.3 million new cases of cancer were diagnosed in 2020 all over the world, and from the total number of diagnosed cancer cases, over 10.1 million cases were reported in males and 9.3 million cases were reported in females. Additionally, according to the 2021 report of the Global RA Network, more than 350 million people are living with arthritis around the world, and its burden is expected to increase owing to various factors, one of which is the rising burden of the geriatric population around the globe. Thus, the growing prevalence of chronic diseases is expected to surge the demand for effective treatment, thereby boosting the growth of the generic drug market over the forecast period.
Furthermore, the geriatric population is more susceptible to chronic diseases, which increases the demand for effective treatment options for prevention and treatment, thereby boosting the market's growth. According to the WHO data for 2021, the proportion of the global population aged 60 and above will nearly double from 12% to 22% between 2015 and 2050. By 2050, 80% of the world's elderly will live in low- and middle-income countries. The population is aging considerably more quickly than in the past, and this demographic is also more likely to suffer from chronic diseases, which will likely increase demand for generic drugs and accelerate market expansion.
Additionally, various organic and inorganic strategies adopted by the key market players are expected to support the growth of the market. In February 2022, Jamp Pharma Group launched Guanfacine XR, a generic version of the reference product INTUNIV XR from Takeda Canada Inc. that treats attention deficit hyperactivity disorder (ADHD). Moreover, in October 2021, The Yangtze River Pharmaceutical Group in China submitted the first marketing application for eslicarbazepine acetate, a class 3 generic medication used to treat epileptic patients. In addition, in July 2021, Generic Ferumoxytol, an injectable drug used to treat iron deficiency anemia (IDA), was launched in the United States by Sandoz, a global leader in generic and biosimilar medicines.
Hence, based on the aforementioned factors, the market is expected to grow significantly during the forecast period. However, stringent government regulation may restrain the market over the forecast period.
Generic Drugs Market Trends
Oral Segment is Expected to Hold Significant Share in the Market Over the Forecast Period
Oral generics are the simplest, most convenient, and safest means of drug administration. They are convenient for repeated and prolonged use and can be self-administered and pain-free; hence, they are the most used and manufactured form of drugs.The growing launches and collaborations are expected to boost the segment's growth. In January 2022, the Medicines Patent Pool (MPP) announced that it had signed agreements with 27 generic manufacturing companies for the manufacturing of the oral COVID-19 antiviral medication molnupiravir and its supply in 105 low- and middle-income countries. In February 2022, Oakrum Pharma, LLC, in collaboration with ANI Pharmaceuticals, announced that the USFDA had approved the ANDA for a generic version of Cystadane1 (betaine anhydrous for oral solution) Powder in a 180-gram bottle and granted competitive generic therapy (CGT) 180 days of exclusivity. Such launches increase the sales and manufacture of oral generic products, boosting the segment's growth.
In addition, in October 2021, Merck & Co. signed a licensing agreement with the United Nations-backed Medicines Patent Pool (MPP) that will allow more companies to manufacture generic versions of its experimental oral antiviral COVID-19 treatment. Such collaborations are likely to boost the segment's growth over the coming years.
Thus, owing to the factors mentioned above, the segment is expected to expand its growth over the forecasted period.
Asia-Pacific is Expected to Registered Healthy CAGR in the Market and Expected to do Same in the Forecast Period
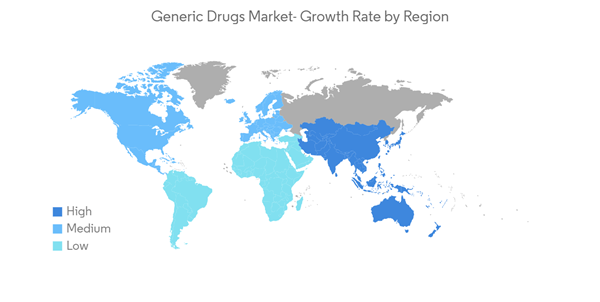
Asia-Pacific is anticipated to witness the fastest-growing trend in the global generic market owing to increased awareness among people related to medical disorders and the growing aging population in the region. Countries like India and China in the Asia-Pacific region contribute more than the other nations. According to a study published by the International Institute for Population Sciences (IIPS), in January 2021, approximately 75 million Indians over the age of 60 had some form of chronic disease. About 45 million people have cardiovascular disease and hypertension, and about 20 million suffer from diabetes. Thus, the high prevalence of chronic diseases in the country will boost the demand for cost-effective therapeutics, thereby driving the studied market.According to the Indian Pharmaceutical Industry Report published in November 2021 by IBEF, India is the world's top supplier of generic pharmaceuticals. The Indian pharmaceutical industry supplies 40% of the generic demand in the United States and 25% of all pharmaceuticals in the United Kingdom.
Strategic and non-strategic initiatives such as partnerships, mergers, and acquisitions by key market players are further expected to contribute to market growth. For instance, in January 2022, Lupin signed a partnership agreement with Shenzhen Foncoo Pharmaceutical Co. to bring high-quality generic and complex generic medicines to patients in India. Further, in October 2021, Dr. Reddy's Laboratories launched an anti-cancer drug in the Chinese market through its joint venture, Kunshan Rotam Reddy Pharmaceutical Co. Ltd. (KRRP). The drug is a therapeutic equivalent generic version of Zytiga, which is owned by Johnson & Johnson.
Therefore, owing to the aforementioned factors, the Asia-Pacific region is expected to show growth at a faster pace over the forecast period.
Generic Drugs Industry Overview
The global generic drugs market is highly competitive, with many players dominating the market. The market players are adopting strategies such as rising R&D investment, mergers, acquisitions, and product innovations to sustain the increasing market rivalry. The key market players include Mylan NV, Eli Lilly and Company, GlaxoSmithKline PLC, Pfizer Inc., Sun Pharma, Novartis, and Sanofi.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Mylan (Viatris Inc.)
- Abbott Laboratories
- Teva Pharmaceutical Industries Limited
- Eli Lilly and Company
- STADA Arzneimittel AG
- GlaxoSmithKline PLC
- Baxter International Inc.
- Pfizer Inc.
- Sanofi
- Novartis AG (Sandoz International)
- Sun Pharma
- AbbVie Inc. (Allergan)