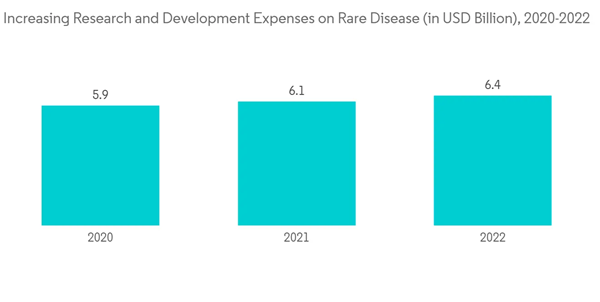
The COVID-19 pandemic impacted the rare disease genetic testing market. The rising number of COVID-19 cases resulted in global healthcare services shifting their resources toward COVID-19 care, which impacted patients with rare diseases. For instance, an article published in the Journal of Public Health, in March 2022, reported that there are between 5,000 and 8,000 rare diseases, most of them with a genetic basis. The article also reported that these rare diseases affect approximately 400 million people worldwide. Many ongoing research projects and clinical trials for rare and genetic diseases were stalled to avoid the transmission of COVID-19 among the patients and research staff. Thus, the pandemic led to a lack of healthcare services and cancellation of appointments, resulting in the underdiagnosis of rare disease patients. However, with the declining cases of COVID-19, the market started to recover at the pre-pandemic levels and is expected to continue the same over the forecast period.
The factors driving the growth of the market are expansion of the patient registry for rare diseases, developing genetic testing technologies, and increasing government initiatives for rare diseases.
The increasing number of available registries for rare diseases is driving the growth of the market. The purpose of rare disease patient registries is to better promote and support patient-focused rare disease research, which improves the foundation for therapy development and scientific understanding of a rare disease. For instance, an article published in March 2022 in the Journal Frontiers in Endocrinology reported that patient registries can fulfil multiple purposes for rare disease research, by improving the knowledge about natural history and variant disease courses and evaluating the safety, effectiveness, and long-term health benefits of preventive care programs, diagnostic strategies, and therapies, such as orphan drugs. Thus, patient registries are effective, convenient, and cost-efficient tools to support documentation of the natural history of a disease, centering patients as research partners for use within rare diseases. The rising number of patient registries is also driving the growth of the studied market.
In February 2022, Bionano Genomics launched a strategic initiative called Rare Undiagnosed Genetic Disease (RUGD) for the 350 million people globally living with rare diseases. Bionano's RUGD initiative includes its suite of product offerings, supporting educational awareness, working toward the development of research grants in this area, and supporting professional societies with a shared mission of improving RUGD patient care and management. As part of Bionano's RUGD initiative, the company has committed to providing three years of financial support for the ACMG Foundation for Genetic and Genomic Medicine (ACMGF) and its Next Generation Fellowship & Residency Training Awards Program in genetics and genomics. Thus, such initiatives are driving the growth of the studied market.
The new genetic testing technologies and the increasing government initiatives for rare disease testing are further propelling the growth of the studied market. For instance, in June 2022, the US FDA revealed its program for rare neurodegenerative diseases, a five-year strategy for extending and refining the lives of people suffering from rare diseases, by evolving the progress of effective and safe medical products and enabling patient access to innovative treatments.
Similarly, in January 2021, the UK Rare Disease Framework was launched, which focused on faster diagnosis, awareness, care, and access to treatment for 3.5 million rare disease patients over five years. Hence, the launch of new technologies, such as NGS-based genetic testing, and the increased demand for diagnosis and government initiatives are contributing to the growth of the rare disease genetic testing market.
Thus, due to the expanding patient registry for rare diseases and increasing government initiatives for the same, as well as the development of new genetic testing technologies, the studied market is expected to witness significant growth over the forecast period. However, a lack of awareness regarding rare diseases and technical challenges associated with genetic tests and data management may slow down the growth of the market.
Rare Disease Genetic Testing Market Trends
Whole Exome Sequencing (WES) is Expected to Witness a Significant Growth During the Forecast Period
The whole-exome sequencing segment is expected to witness significant growth over the forecast period due to the increasing applications of WES in clinical diagnosis and the growing demand for the diagnosis of rare diseases, along with the increasing R&D in the field of genomics and next-generation sequencing and increasing demand for personalized medicine.The high adoption of the whole-exome sequencing genetic testing tool is driving the growth of the segment. This technique targets only exons, which make 1-2% of the whole genome and contains 80% of all disease-causing mutations. Such continuous technological developments are expected to provide accurate and rapid results. For instance, an article published by the BMJ Journal, in November 2021, reported that WES is available for selected patients for the routine diagnosis of rare childhood genetic diseases. The article also reported that next-generation sequencing allows hundreds or thousands of genes to be sequenced in a short period at a much lower cost.
The strategic activities of key players are estimated to further drive the segment over the forecast period. For instance, in October 2022, BGI Australia's lab achieved accreditation from the NATA to perform clinical WES in Australia. This accreditation will facilitate BGI Australia to provide services to clinical laboratories, hospitals, and other partners to detect changes in the exome that contributes to rare genetic and pediatric diseases.
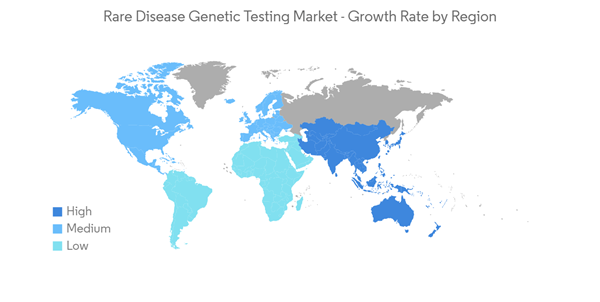
North America Expected to Witness a Significant Growth During the Forecast Period
North America is expected to witness significant growth over the forecast period due to the high prevalence of rare diseases, a large number of disease registries, the presence of a substantial number of R&D facilities for ultra-rare diseases, and extensive investments in the diagnosis of disease. For instance, in 2021, the Genetic Rare Diseases Information Center (GARD) reported that there were about 7,000 known rare disease cases, which accounted for about 1 in 10 people, and 30 million people in the United States had a rare disease during 2020-2021. Thus, the high prevalence of rare diseases is increasing the demand for advanced rare disease testing devices, thereby propelling the growth of the market in the region.The government initiatives to provide financial support and funding for the ongoing clinical trials for drug testing to overcome the high prevalence of these diseases in the United States are also supporting the market's growth. For instance, in October 2020, the US FDA carried out the Orphan Drug Act and awarded over USD 16 million for six clinical trial research studies to industry and academia for the next four years. Thus, the increasing government funding is supporting research for new drug trials and driving the growth of the market in the region.
The strategic partnerships among key market players, new product launches, and mergers and acquisitions have also propelled the market's growth in the region. For instance, in November 2021, Genomenon collaborated with Alexion and AstraZeneca Rare Disease to make the treatment and diagnosis of rare diseases more readily available. The goal of the collaboration is to empower the genetic testing laboratories with the data they need for the diagnosis of rare diseases.
Along with rising prevalence and increased approvals, the supportive healthcare infrastructure, government initiatives, and technological advancements in North America are expected to drive the market. For instance, in April 2021, Centogene entered a partnership with Takeda Pharmaceutical Company Limited to diagnose patients through access to Centogene's genetic disease testing capabilities. Thus, such technological developments are fueling the growth of the market in the region.
Due to the high prevalence of rare diseases, a large number of disease registries, the presence of a substantial number of R&D facilities for ultra-rare diseases, and extensive investments in the diagnosis of diseases, the region is expected to witness significant growth over the forecast period.
Rare Disease Genetic Testing Industry Overview
The rare disease genetic testing market is highly competitive and fragmented due to the presence of many players. These players are present globally and regionally, which include Quest Diagnostics Incorporated, Eurofins Scientific Inc., SAmbry Genetics Corporations, PerkinElmer Genetics Inc., Baylor Miraca Genetics Laboratories LLC, and Beijing Genomics Institute Ltd.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Quest Diagnostics Incorporated
- Centogene NV
- Invitae Corporation
- 3billion Inc.
- ARUP Laboratories
- Eurofins Scientific Inc.
- Strand Life Sciences Private Limited
- Ambry Genetics Corporations
- PerkinElmer Genetics Inc.
- Macrogen Inc.
- Baylor Miraca Genetics Laboratories, LLC
- Color Health Inc.
- Beijing Genomics Institute Ltd