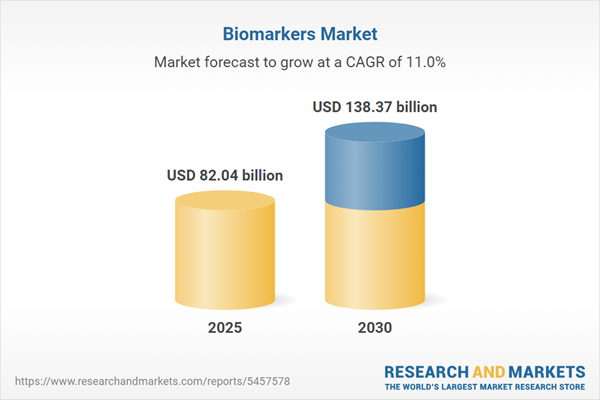
Biomarkers Market Size:
The Biomarkers Market is expected to grow from USD 82.039 billion in 2025 to USD 138.365 billion in 2030, at a CAGR of 11.02%.The diagnostic market is experiencing unprecedented demand driven by the critical need for early disease detection capabilities. Traditional diagnostic tools, while focused on accuracy and consistency, are being superseded by advanced systems that deliver precise results during early disease stages. This evolution is particularly significant in cancer diagnostics, where early detection presents substantial technical challenges but offers dramatically improved patient outcomes.
Biomarkers represent non-invasive diagnostic solutions that address these market requirements through sophisticated molecular identification and analysis capabilities. The integration of biomarkers with modern imaging technologies and data management systems creates comprehensive diagnostic platforms that meet evolving market expectations while reducing patient exposure to radiation during CT scans and MRI procedures.
Primary Growth Drivers
Companion Diagnostics Development
Medical companion diagnostics and biomarkers have emerged as increasingly critical components enabling enhanced diagnosis, treatment selection, and disease monitoring across multiple therapeutic areas. This approach facilitates precise patient identification for specific drug therapies and treatment protocols, optimizing therapeutic outcomes while minimizing adverse effects.Diagnostic tests are frequently developed in conjunction with specific pharmaceutical treatments, creating integrated therapeutic approaches that improve patient care. Biomarkers play essential roles in companion diagnostic development by identifying molecular targets that serve as drug interaction points, enabling precision medicine approaches that tailor treatments to individual patient characteristics.
Pharmaceutical companies increasingly partner with diagnostic developers to create companion diagnostic solutions. These collaborative efforts focus on developing targeted therapeutics alongside corresponding diagnostic tools, ensuring optimal patient selection and treatment monitoring capabilities throughout the therapeutic process.
Diagnostic Biomarker Market Expansion
Diagnostic biomarker testing has gained significant prevalence through technological advancement in clinical laboratory testing, imaging examinations, and clinical patient management systems. Technological progress in genomics and proteomics fields positions diagnostic biomarker technology to revolutionize clinical research, medical practice, and drug development processes.Biomarkers provide valuable support for drug development initiatives while enabling diagnosis of critical diseases and supplying essential clinical trial information. These molecular indicators allow pathologists to identify diseases more rapidly and accurately than traditional diagnostic methods, improving patient outcomes and healthcare efficiency.
Artificial intelligence-driven technologies are increasingly integrated into biomarker detection systems, enhancing diagnostic accuracy and enabling automated analysis of complex biological data. The MedTech industry demonstrates substantial investment commitment to biomarker development, particularly in Asia-Pacific regions where market opportunities continue expanding.
Market Applications and Utility
Biomarker applications encompass risk assessment, molecular diagnostics, disease diagnosis, DNA fingerprinting, and various molecular analysis applications. The increasing demand for safe and effective treatment dosing makes biomarkers essential tools for pharmaceutical companies developing targeted therapies and personalized medicine approaches.Biomarker utilization eliminates complex data preparation, testing, and assay development requirements in drug development processes, streamlining pharmaceutical research and reducing development timelines. The growing prevalence of cancer and cardiovascular diseases, combined with increasing investment by major market participants in biomarker production, projects sustained demand growth throughout the forecast period.
Market Challenges and Constraints
Validation Complexities
Biomarker validation presents significant challenges requiring evaluation of performance characteristics including sensitivity, specificity, and reproducibility metrics. Establishing biomarkers as reliable tools for supporting biopharmaceutical investment decisions demands comprehensive validation processes that can be costly and time-consuming, particularly for smaller organizations with limited resources.Inherent variability in biomarker levels and molecular heterogeneity creates additional validation challenges that must be addressed before clinical implementation. Demonstrating clinical validity and utility remains essential for regulatory approval and physician adoption, but the lack of standardized protocols makes data collection processes expensive and prolonged.
Competitive Landscape and Strategic Partnerships
Market growth stems from personalized medicine advancement, technological progress in disease diagnosis, and increasing preference for biomarkers in drug discovery and development. Strategic collaborations between pharmaceutical companies and diagnostic developers drive innovation and market expansion through shared expertise and resource optimization.Recent partnerships focus on identifying novel biomarkers for treatment response prediction and developing advanced genomics platforms for cancer immunology applications. New product introductions result from collaborative efforts that combine pharmaceutical expertise with diagnostic technology capabilities, creating integrated solutions that address complex clinical requirements while advancing precision medicine initiatives across multiple therapeutic areas.
Key Benefits of this Report:
- Insightful Analysis: Gain detailed market insights covering major as well as emerging geographical regions, focusing on customer segments, government policies and socio-economic factors, consumer preferences, industry verticals, and other sub-segments.
- Competitive Landscape: Understand the strategic maneuvers employed by key players globally to understand possible market penetration with the correct strategy.
- Market Drivers & Future Trends: Explore the dynamic factors and pivotal market trends and how they will shape future market developments.
- Actionable Recommendations: Utilize the insights to exercise strategic decisions to uncover new business streams and revenues in a dynamic environment.
- Caters to a Wide Audience: Beneficial and cost-effective for startups, research institutions, consultants, SMEs, and large enterprises.
What do businesses use these reports for?
Industry and Market Insights, Opportunity Assessment, Product Demand Forecasting, Market Entry Strategy, Geographical Expansion, Capital Investment Decisions, Regulatory Framework & Implications, New Product Development, Competitive IntelligenceReport Coverage:
- Historical data from 2022 to 2024 & forecast data from 2025 to 2030
- Growth Opportunities, Challenges, Supply Chain Outlook, Regulatory Framework, and Trend Analysis
- Competitive Positioning, Strategies, and Market Share Analysis
- Revenue Growth and Forecast Assessment of segments and regions including countries
- Company Profiling (Strategies, Products, Financial Information, and Key Developments among others.
Global Biomarkers Market Segments:
By Type
- Safety Biomarkers
- Validation Biomarkers
- Efficacy Biomarkers
By Application
- Drug Discovery and Development
- Diagnostics
- Disease Risk Assessment
- Personalized Medicine
- Other
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- Germany
- France
- United Kingdom
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- China
- India
- Japan
- South Korea
- Indonesia
- Thailand
- Others
Table of Contents
Companies Mentioned
- Cisbio
- MESO SCALE DIAGNOSTICS, LLC
- PerkinElmer Inc.
- Bio-Rad Laboratories
- QIAGEN
- Signosis Inc.
- Thermo Fisher Scientific
- Siemens
- Roche Diagnostics
- Agilent
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 149 |
| Published | August 2025 |
| Forecast Period | 2025 - 2030 |
| Estimated Market Value ( USD | $ 82.04 billion |
| Forecasted Market Value ( USD | $ 138.37 billion |
| Compound Annual Growth Rate | 11.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |