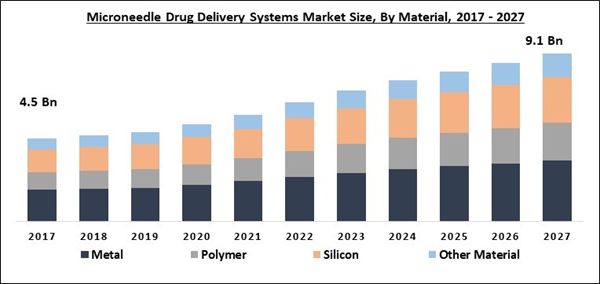
The Global Microneedle Drug Delivery Systems Market size is expected to reach $9.1 billion by 2027, rising at a market growth of 7.8% CAGR during the forecast period. The microneedle drug delivery device is designed with the arrangement of hundreds of microneedles in a small patch order to pass an ample supply of the drug to provide a needed therapeutic response. This device helps in the direct delivery of the drug into the viable epidermis without coming in contact with blood vessels and nerve fibers that are present in the dermal layers. Silicon, carbohydrate, silica glass, polymers, and ceramic are some of the materials utilized in the manufacturing of microneedle. Generally, microneedles are hundreds of microns long and up to 1 to 50 mm wider at the tip and almost 50-300 mm at the base.
The factors accelerating the growth of the microneedle drug delivery system market are increasing the demand for a safer alternative to conventional hypodermic injections, and rising research & development initiatives. Additionally, the microneedle drug delivery systems have excessively been adopted due to the emerging safety issues that have short and long-term implications for patients, regulators, healthcare administrators, and clinicians. As stated by the Centers for Disease Control and Prevention, an innovative patch can facilitate the administration of the vaccine to people suffering from measles and other vaccine-preventable diseases.
COVID-19 Impact Analysis
The healthcare sector was severely affected and faced numerous challenges. Except for COVID patient, all other treatments were kept on hold to minimize the possibility of viral transmission. The demand for medical devices and equipment decreased, which led to a declined demand for microneedle drug delivery systems in the market. However, as a number of patients' treatments are on hold, it is anticipated that the demand and growth of different markets is expected to increase again in the post-COVID period, including the microneedle drug delivery systems market.
Market Growth Factors:
The growth in the occurrence of premature birth
The surge in the occurrence of pre-mature birth in emerging nations and the increase in malnutrition among children are expected to boost the demand for the microneedle drug delivery systems market during the forecasting period. A significant number of premature births are noted in emerging nations such as India, Indonesia, Nigeria, and the Philippines. Additionally, the surge in the acceptance of microneedle drug delivery systems for premature infants results in better neurological growth, enhanced intrauterine nutrient deposition, and lower chances of complications, hence enhancing their possibility of survival.
Higher prevalence of different chronic diseases
Various kinds of diseases like head & neck cancer, esophageal cancer, Crohn's, ulcerative colitis, gastric cancer, and laryngeal cancer can have an impact on oral food consumption among the patients. Due to their increasing occurrence, the microneedle drug delivery systems market is expected to be propelled in the upcoming years. In addition, the increasing number of people suffering from various pediatric diseases such as asthma, tuberculosis, leukemia, chickenpox, bronchitis, and anemia combined with the surge in the geriatric population that are more vulnerable to chronic diseases is expected to bolster the demand for microneedle drug delivery systems over the coming years.
Market Restraining Factor:
Length and size of microneedles leads to severe pain
Microneedles are available in different size, length and type. The longer microneedles may lead to excessive pain during the procedure. As the microneedles are longer in length, they touch the deepest layer of skin, which causes severe pain to the patient. Thus, excessive usage of microneedles for the delivery of vaccine or drug may lead to cause severe pain to the patient, which is anticipated to decrease the demand for the microneedle drug delivery systems in the coming years.
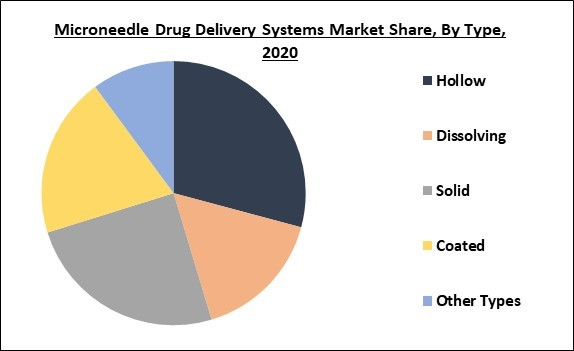
Type Outlook
Based on Type, the market is segmented into Hollow, Dissolving, Solid, Coated, and Other Types. The dissolving microneedle segment is anticipated to grow at the fastest CAGR over the forecast years. The delivery of active ingredients through dissolving microneedle is less painful procedure as compared to the syringe. Once the needle is inserted in the skin, it is not taken out as it gets automatically dissolved in the skin.
Material Outlook
Based on Material, the microneedle drug delivery systems market is segregated into Silicon, Metal, Polymer, and Others. In 2020, the metal material segment acquired the largest market share of the microneedle drug delivery systems market. The most commonly used metals are titanium and stainless steel. Other types of metals that are also used are palladium-cobalt alloys, palladium, and nickel. These metals comprise biocompatibility and mechanical qualities.
Application Outlook
Based on Application, the market is segmented into Drug delivery, Cancer Therapy, Vaccine delivery, Pain Management, Dermatology and Other Applications. In 2020, the drug delivery segment procured the maximum revenue share. The insulin hormone is made up of peptides. Patients who have high blood sugar levels are suggested to take insulin. Various experiments were conducted by different scientists and these experiments confirmed that delivery of insulin through microneedle is the best way, and helps in producing proper biological effects and maintaining blood glucose levels.
Regional Outlook
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. Asia-Pacific is anticipated to exhibit fastest growth rate over the forecast years. The emergence of the COVID-19 has highlighted the vulnerabilities in the regional healthcare sector. Additionally, a lack of interconnection is also witnessed between economic stability and health security.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include AdminMed nanoBioSciences LLC, Becton, Dickinson and Company, Zosano Pharma Corporation, Raphas co. ltd., Nanopass Technologies Ltd., Corium International, Inc., Gurnet Point Capital), Valeritas, Inc. (Zealand Pharma), Nitto Denko Corporation, Endoderma Ltd., and Innoture Medical Technology Limited.
Recent Strategies Deployed in Microneedle Drug Delivery Systems Market
Partnerships, Collaborations and Agreements:
- Oct-2021: BD came into a partnership with Aptar Pharma, a global leader in drug delivery solutions and services. Under this partnership, BD aimed to introduce BD SCF Premium Coat Plunger Stopper, a syringe plunger stopper developed to support the injection of biologics into subcutaneous tissue with a 1mlL pre-filled syringe.
- Aug-2020: Zosano Pharma Corporation signed an agreement with Mitsubishi Tanabe Pharma Corporation, a global pharmaceutical company. Under this agreement, Mitsubishi Tanabe Pharma Corporation is expected to enable Zosano Pharma Corporation to integrate a proprietary transdermal microneedle drug delivery platform with Mitsubishi Tanabe Pharma Corporation’s drug development candidate and to display the wide applicability of Zosano Pharma Corporation platform to provide the difference in the lives of patients when integrated with pharmaceutical products, which is expected to fulfill considerable unmet medical requirements.
- Aug-2020: Zosano Pharma Corporation formed a partnership with EVERSANA, a leading provider of commercial services to the life science industry. This partnership is expected to distribute and commercialize Qtrypta across the United States.
- Apr-2020: NanoPass entered into a partnership with Pharma Partners, the leading vaccine and Immunotherapy Company. Under this partnership, NanoPass is expected to share its proprietary MicronJet microneedle device to allow the designing of an effective, affordable, and safe vaccine.
- Jan-2020: Corium received approval for ADLARITY, donepezil transdermal system, from the U.S. Food and Drug Administration. Through this approval, ADLARITY is expected to treat patients suffering from mild, moderate, and severe Alzheimer’s disease.
Acquisitions and Mergers:
- Jun-2020: BD took over Straub Medical, a privately-held company. Under this acquisition, the company is expected to offer the latest technologies in both thrombectomy & atherectomy and is expected to further improve the company's robust portfolio of angioplasty & scoring balloons, stent grafts, stents, drug-coated, and CTO crossing products for the treatment of venous disease and PAD.
Geographical Expansions:
- Oct-2021: BD expanded its geographical reach in the US by strengthening its government access to safe injection devices by increasing domestic supply and manufacturing capacity. The latest manufacturing lines in the US is expected to offer the US government priority access to hundreds of millions of syringes and needles which is expected to accelerate the supply chain.
- Jul-2020: Zealand Pharma expanded its geographical reach in Boston by the opening of its new U.S. headquarters. By opening headquarters, the company is expected to shift its commercial operations to the new headquarter as the company is expected to commercialize pipeline in the US.
Scope of the Study
Market Segments Covered in the Report:
By Type
- Hollow
- Dissolving
- Solid
- Coated,
- Other Types
By Material
- Metal
- Polymer
- Silicon
- Other Material
By Application
- Drug delivery
- Cancer Therapy
- Vaccine delivery
- Pain Management
- Dermatology
- Other Applications
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- AdminMed nanoBioSciences LLC
- Becton, Dickinson and Company
- Zosano Pharma Corporation
- Raphas co. ltd.
- Nanopass Technologies Ltd.
- Corium International, Inc. (Gurnet Point Capital)
- Valeritas, Inc. (Zealand Pharma)
- Nitto Denko Corporation
- Endoderma Ltd.
- Innoture Medical Technology Limited
Unique Offerings from the Publisher
- Exhaustive coverage
- The highest number of market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- AdminMed nanoBioSciences LLC
- Becton, Dickinson and Company
- Zosano Pharma Corporation
- Raphas co. ltd.
- Nanopass Technologies Ltd.
- Corium International, Inc. (Gurnet Point Capital)
- Valeritas, Inc. (Zealand Pharma)
- Nitto Denko Corporation
- Endoderma Ltd.
- Innoture Medical Technology Limited