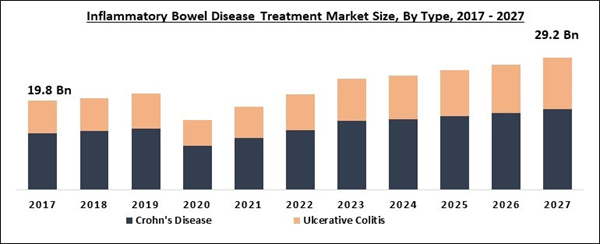
The Global Inflammatory Bowel Disease Treatment Market size is expected to reach $29.2 billion by 2027, rising at a market growth of 8.1% CAGR during the forecast period. Inflammatory bowel disease refers to a group of intestinal disorders that leads to chronic inflammations like swelling and pain in the intestines. Few of the known inflammatory bowel disease are Ulcerative colitis and Crohn’s disease. The effect of both types of inflammatory bowel disease is witnessed on the digestive system. There are various treatments available that only help to slow down the progress of the disease. The kind of treatment required for inflammatory bowel disease largely depends upon symptoms and the type of IBD. Moreover, the prescribed medicines assist patients in controlling inflammation.
The key factor contributing to the growth of the market is the growing prevalence of ulcerative disease and Crohn’s disease around the world. Additionally, the presence of strong pipeline drugs like risankizumab, ustekinumab, tofacitinib, & upadacitinib, and the growing adoption of biologics for the inflammatory bowel disease treatment are projected to accelerate the growth of the market in the upcoming period. However, the outbreak of the COVID-19 has an adverse effect on the growth of the market as a rapid decrease was observed in the diagnosis of inflammatory bowel disease, owing to the imposition of lockdown in several countries around the world.
COVID-19 Impact Analysis
The outbreak of the COVID-19 pandemic had a negative impact across the world. During the Quarter 2 of 2020, there was a rapid increase witnessed in the number of COVID-19 patients due to which, WHO declared it as a Public Health Emergency. Governments across the world formed rules to reduce the impact of the COVID-19. Lockdown was imposed that led to the shutdown of businesses, which had a negative impact on the economies of nations. Traveling was banned that disrupted the supply chains. People were forced to stay at their homes and maintain social distance.
Market Growth Factors:
High government spending on IBD treatment research
As the prevalence of inflammatory bowel disease is increasing rapidly, governments across several nations are focusing on finding efficient and less painful treatments for IBD patients. Thus, governments are highly investing in research and development activities to develop new products that will provide relief to patients suffering from inflammatory bowel disease. Moreover, the government is also providing reimbursement policies to patients due to which, a large number of patients are adopting IBD treatment.
Rapid urbanization led to an unhealthy lifestyle
As the world is evolving, most of the nations are shifting towards urbanization. People residing in different nations are largely following modern culture and trending activities. People are also inclining towards a luxurious life, which has given rise to an unhealthy lifestyle. In addition, the advent of advanced technologies has made people lazy and inactive. Additionally, people are adopting unhealthy activities such as smoking and consuming alcohol. The unhealthy lifestyle has resulted in giving rise to various health-related problems like inflammatory bowel disease.
Market Restraining Factor:
Strict regulatory policies for IBD drugs
The guidelines for the British Society of Gastroenterology were developed on the management of inflammatory bowel disease in youngsters. The major UK authorities of Ulcerative colitis and Crohn's disease healthcare were involved in the development of guidelines. These guidelines were imposed at the time of rapid change in several factors of inflammatory bowel disease. The imposition of stringent guidelines has created barriers to the growth of the inflammatory bowel disease treatment market.
Type Outlook
Based on Type, the market is segmented into Crohn's Disease and Ulcerative Colitis. In 2020, the Crohn's Disease segment acquired the largest revenue share of the inflammatory bowel disease treatment market and is expected to continue the progress during the forecast period. The major factors accelerating the growth of the segment are the growing rate of biologics prescription and the high prevalence rate.
Distribution Channel Outlook
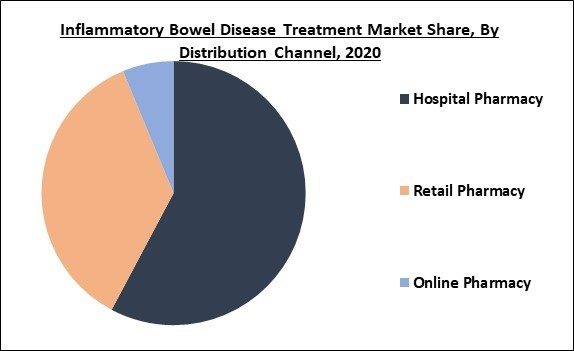
Based on Distribution Channel, the market is segmented into Hospital Pharmacy, Retail Pharmacy and Online Pharmacy. In 2020, the hospital pharmacy segment collected the maximum revenue share of the inflammatory bowel disease treatment market and is expected to display similar kind of trend even during the forecast years. The factors contributing to the growth of the segment are rapidly growing hospitalization of inflammatory bowel disease patients and higher cost of biologics.
Route of Administration Outlook
Based on Route of Administration, the market is segmented into Injectable and Oral. The oral segment is anticipated to display a prominent CAGR during the forecasting years. Factors such as the presence of robust oral pipeline products to treat patients suffering from inflammatory bowel disease and the approval of JAK inhibitors for inflammatory bowel disease treatment are responsible for the growth of this segment. Moreover, the forthcoming launch of S1P modulators and JAK inhibitors will propel the growth of the market over the forecast period.
Drug Class Outlook
Based on Drug Class, the market is segmented into TNF inhibitors, Anti-integrin, IL inhibitors, Corticosteroids, JAK inhibitors, Aminosalicylates and Others. The drug segment like aminosalicylates, corticosteroids, and anti-integrin are displaying a prominent share in the inflammatory bowel disease treatment market. Aminosalicylates refer to the first-line therapy for inflammatory bowel disease treatment. JAK inhibitors segment is expected to showcase the fastest growth rate during the forecasting years.
Regional Outlook
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. Asia-Pacific is expected to exhibit the fastest CAGR of inflammatory bowel disease treatment during the forecasting years. The growth of the regional market is expected to be driven by the increasing novel drug approval, the growing prevalence of ulcerative colitis and Crohn's disease across the region, and increasing prescription of biosimilars. Based on a study by NCBI, in comparison to women, the chances of Crohn's disease are higher in men.
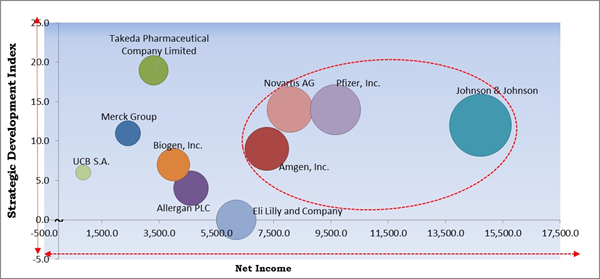
The major strategies followed by the market participants are Approvals. Based on the Analysis presented in the Cardinal matrix; Pfizer, Inc., Amgen, Inc., Novartis AG, and Johnson & Johnson are the forerunners in the Inflammatory Bowel Disease Treatment Market. Companies such as Takeda Pharmaceutical Company Limited, UCB S.A., Allergan PLC are some of the key innovators in the market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Allergan PLC (AbbVie), Johnson & Johnson, Merck Group, Amgen, Inc., Eli Lilly and Company, Biogen, Inc., Pfizer, Inc., Novartis AG, Takeda Pharmaceutical Company Limited, and UCB S.A.
Recent Strategies Deployed in Inflammatory Bowel Disease Treatment Market
Partnerships, Collaborations and Agreements:
- Jul-2021: Merck collaborated with B Braun, a German medical and pharmaceutical device company. Together, the companies aimed to treat inflammatory diseases utilizing neurostimulator devices. Moreover, the companies is expected to allow multi-channel selective stimulation, could solve the important challenges, and provide neurostimulator treatment to patients that are suffering from chronic inflammatory diseases.
- Sep-2020: Merck teamed up with Seattle Genetics, an American biotechnology company. Together, the companies is expected to introduce two new strategic oncology collaborations. These two strategic collaborations is expected to allow Seattle Genetics to further expand Merck’s broad oncology offerings and pipeline, and to continue Seattle Genetics efforts to enhance and extend the lives of various patients suffering from cancer.
- Jul-2020: UCB signed an agreement with Ferring Pharmaceuticals, a Swiss multinational biopharmaceutical company. Under this agreement, the companies aimed to commercialize the prefilled syringe formulation of CIMZIA across the United States, particularly for the treatment of Crohn's disease.
Acquisitions and Mergers:
- Apr-2021: Merck took over Pandion Therapeutics, a clinical-stage biotechnology company. This acquisition is expected to cater all the requirements of patients living with autoimmune diseases.
- Mar-2021: Amgen signed an agreement to acquire Rodeo, a privately held biopharmaceutical company. Under this acquisition, the company is expected to design first-in-class therapeutics for patients.
- Feb-2020: Takeda took over PvP Biologics, a biotechnology company. Under this acquisition, the company is expected to use TAK-062 for the treatment of uncontrolled celiac disease.
Geographical Expansions:
- May-2021: Amgen expanded its geographical reach in Canada by introducing AMGEVITA for the treatment of 11 chronic inflammatory conditions. Through this expansion, the company aimed to use AMGEVITA to expand the number of treatment options available for Canadians suffering from chronic inflammatory diseases.
Approvals and Trials:
- Sep-2021: Takeda Pharmaceutical Company received approval to manufacture and market Alofisel development code Cx601 from the Japan Ministry of Health, Labour and Welfare. The company is expected to use this development code for the treatment of complex perianal fistulas in patients with non-active or mildly active luminal Crohn’s disease.
- Aug-2021: Novartis received approval for Cosentyx from the China National Medical Products Administration. Through this approval, the company aimed to use Cosentyx for the treatment of moderate-to-severe plaque psoriasis in pediatric patients who are candidates for systemic therapy or phototherapy.
- Aug-2021: UCB received approval for BIMZELX from the European Commission. The company is expected to use BIMZELX for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
- Aug-2021: AbbVie received approval for RINVOQ from the European Commission. The company aimed to use RINVOQ for the treatment of moderate to severe atopic dermatitis across adults and adolescents of 12 years and above, who are candidates for systemic therapy.
- Jun-2021: Novartis got approval for Cosentyx from the U.S. Food and Drug Administration. The company is expected to use Cosentyx for the treatment of moderate to severe plaque psoriasis in pediatric patients, for six years and older who are candidates for phototherapy1 or systemic therapy.
- May-2021: Janssen, a subsidiary of Johnson & Johnson got approval for PONVORY from the European Commission. The company is expected to use PONVORY for the treatment of adult patients with relapsing multiple sclerosis with active disease defined by imaging or clinical features.
- Mar-2021: Janssen, a subsidiary of Johnson & Johnson received approval for PONVORY from the U.S. Food and Drug Administration. The company is expected to use PONVORY to treat adults with relapsing forms of multiple sclerosis, including relapsing-remitting disease, active secondary progressive disease, and clinically isolated syndrome.
- Feb-2021: Pfizer received the approval of supplemental Biologics License Application for PANZYGA from the U.S. Food and Drug Administration. PANZYGA is expected to treat adult patients with a rare neurological disease of the peripheral nerves known as chronic inflammatory demyelinating polyneuropathy.
- Feb-2021: AbbVie got approval for HUMIRA from the U.S. Food and Drug Administration. The company is expected to use HUMIRA for the treatment of moderately to severely active ulcerative colitis in pediatric patients of 5 years and above.
- Aug-2020: Novartis received approval for Kesimpta from the US Food and Drug Administration. Under this approval, the company aimed to use Kesimpta for the treatment of relapsing forms of multiple sclerosis, including relapsing-remitting disease, active secondary progressive disease, and clinically isolated syndrome, in adults.
- Mar-2020: Takeda Pharmaceutical Company got approval for Entyvio from the China National Medical Products Administration. The company aimed to use Entyvio for the treatment of adult patients with moderate to severe active ulcerative colitis or Crohn's disease, who have had a poor response with, lost response to, or were intolerant to conventional therapies or tumor necrosis factor-alpha (TNFα) inhibitors.
Scope of the Study
Market Segments Covered in the Report:
By Type
- Crohn's Disease and
- Ulcerative Colitis
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By Route of Administration
- Injectable
- Oral
By Drug Class
- TNF inhibitors
- Anti-integrin
- IL inhibitors
- Corticosteroids
- JAK inhibitors
- Aminosalicylates
- Others
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Allergan PLC (AbbVie)
- Johnson & Johnson
- Merck Group
- Amgen, Inc.
- Eli Lilly and Company
- Biogen, Inc.
- Pfizer, Inc.
- Novartis AG
- Takeda Pharmaceutical Company Limited
- UCB S.A.
Unique Offerings from the Publisher
- Exhaustive coverage
- The highest number of market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Allergan PLC (AbbVie)
- Johnson & Johnson
- Merck Group
- Amgen, Inc.
- Eli Lilly and Company
- Biogen, Inc.
- Pfizer, Inc.
- Novartis AG
- Takeda Pharmaceutical Company Limited
- UCB S.A.