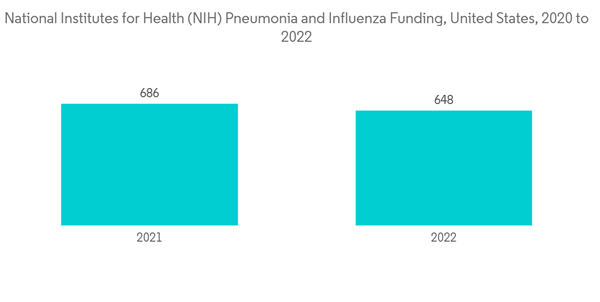
The COVID-19 pandemic has had an impact on the pneumonia treatment market as patients suffering from COVID-19 are highly susceptible to pneumonia, causing a sharp surge in the number of cases of viral pneumonia. As per the NIH updated in September 2022, Hospitalized patients with COVID-19 may acquire common nosocomial infections, such as hospital-acquired pneumonia (including ventilator-associated pneumonia). Moreover, as per the study published in July 2021 by the British medical journal, the COVID-19 pandemic led to an unprecedented surge in hospitalized patients with viral pneumonia. Early diagnosis and treatment of these infections are important for improving outcomes in these patients. Thus, COVID-19 impacted the pneumonia treatment market, owing to the growth in demand for COVID-19-associated pneumonia and the surge in the incidence of pneumonia cases during the commenced period. However, even after the pandemic, the market is expected to see a surge due to an increase in lung injuries in many people with COVID-19. For instance, as per the study published In March 2022, By the Radiological Society of North America, Long-term CT abnormalities were common 1 year after COVID-19 pneumonia.
The major contributing factors to the market growth are the increasing prevalence of pneumonia and increasing product approvals related to the treatment of pneumonia. For instance, as per the UNICEF data updated in August 2021, globally, there are over 1,400 cases of pneumonia per 100,000 children, or 1 case per 71 children every year, with the greatest incidence occurring in South Asia (2,500 cases per 100,000 children) and West and Central Africa (1,620 cases per 100,000 children). Thus, with the increasing cases of pneumonia, the demand for its treatment is also expected to increase, which is further expected to boost the growth of the market over the forecast period.
Moreover, an increasing number of ongoing clinical trials for the development of drug molecules and various approvals by the key players will have a significant impact on the market progression. Currently, different companies operating in the pneumonia therapeutics market, including Merck and GSK, are putting enormous efforts into research and development activities. For instance, in February 2020, Food and Drug Administration accepted the review supplemental new drug application for Recarbrio for the treatment of adults with ventilator-associated and hospital-acquired bacterial pneumonia. In June 2021, the US FDA approved PREVNAR 20, Pfizer’s pneumococcal 20-valent conjugate vaccine for adults ages 18 years or older, for the prevention of invasive disease and pneumonia caused by the 20 Streptococcus pneumonia (pneumococcus) serotypes in the vaccine. Thus, such developments are expected to boost the growth of the pneumonia treatment market over the forecast period.
However, low treatment rates in developing economies are expected to hinder the growth of the market over the forecast period.
Pneumonia Treatment Market Trends
Aminopenicillin Segment is Expected to Exibhit a Steady Growth in the Pneumonia Treatment Market
Aminopenicillin is a group of antibiotics that are usually used for the treatment of upper and lower respiratory tract infections. Amoxicillin, a penicillin-like drug, is the first-line treatment of community-acquired pneumonia infection, according to the Infectious Disease Society of America. Amoxicillin binds to penicillin-binding proteins that activate an autolytic enzyme that destroys the bacterial cell wall and thereby kills the bacterial cell.As per the study published by the National Library of Medicine, in June 2021, over the study period, a favorable trend was seen as aminopenicillin prescriptions (20.1% to 31.8%) increased and prescriptions for macrolides decreased (45.8% to 40.5%). Thus, the adoption of aminopenicillin for the treatment of pneumonia over the other drugs is expected to boost the growth of the segment over the forecast period.
Furthermore, the number of hospital stays is higher in the case of adults, which makes them at a greater risk of hospital-acquired pneumonia. For instance, as per the study published in September 2021 by Med Care, 1.2% of hospitalizations were 21 days or longer. Patients with prolonged hospitalizations were younger and had a greater number of chronic diseases. Thus, the increasing number of hospital stays is expected to increase in the cases of hospital-acquired pneumonia, which is expected to boost the growth of the market over the forecast period.
Thereby, the growing burden of pneumonia, advantages associated with aminopenicillins, and the high number of hospital stays are expected to drive the market in this segment.
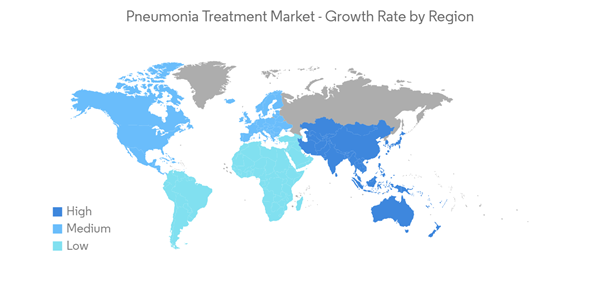
North America is Expected to Hold a Significant Share in the Market and Expected to do Same in the Forecast Period
North America is expected to hold a major market share in the global pneumonia treatment market due to the growing burden of pneumonia infection and increasing product approvals. For instance, as per the study published in August 2021 by NCBI, the estimated worldwide incidence of community-acquired pneumonia varies between 1.5 to 14 cases per 1000 person-years, and this is affected by geography, season, and population characteristics. In the United States, the annual incidence is 24.8 cases per 10,000 adults, with higher rates as age increases. Thus, such an increased burden of community-acquired pneumonia in the country is expected to increase the demand for its treatment which is expected to influence positively on the pneumonia treatment market.Also, the increasing launches by manufacturers to meet the growing demand for innovative products are expected to drive market growth. For instance, in June 2021, the US FDA approved omadacycline, an oral-only dosing regimen, for the treatment of community-acquired bacterial pneumonia. Such factors potentially drive the demand for the adoption of pneumonia treatment in the region.
Thus, all the aforementioned factors are expected to boost the market in the region over the forecast period.
Pneumonia Treatment Market Competitor Analysis
The Pneumonia Treatment Market is fragmented and consists of several major players. Some of the companies which are currently dominating the market are Pfizer Inc., Teva Pharmaceuticals Inc., Abbott, Baxter International, and Viatris.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott
- AbbVie
- Akorn Inc.
- Baxter International Inc.
- Lupin Pharmaceuticals Inc.
- Viatris
- Neopharma
- Novartis AG
- Pfizer Inc.
- Teva Pharmaceuticals Inc.